Antibiotics: Pencillin
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
21 Terms
How does penicillin kill bacteria?
Prevents synthesis of the cross links
which leaves the cell wall too weak to resist osmotic lysis.
How does penicillin prevent synthesis of cell wall cross-links?
Inhibits transpeptidase enzyme by blocking peptide chains attaching to the enzyme
What is an issue with penicillin G (IV form)?
Must be injected as it degrades in the acidic environment of the stomach when given orally.
Describe the structure of penicillin.
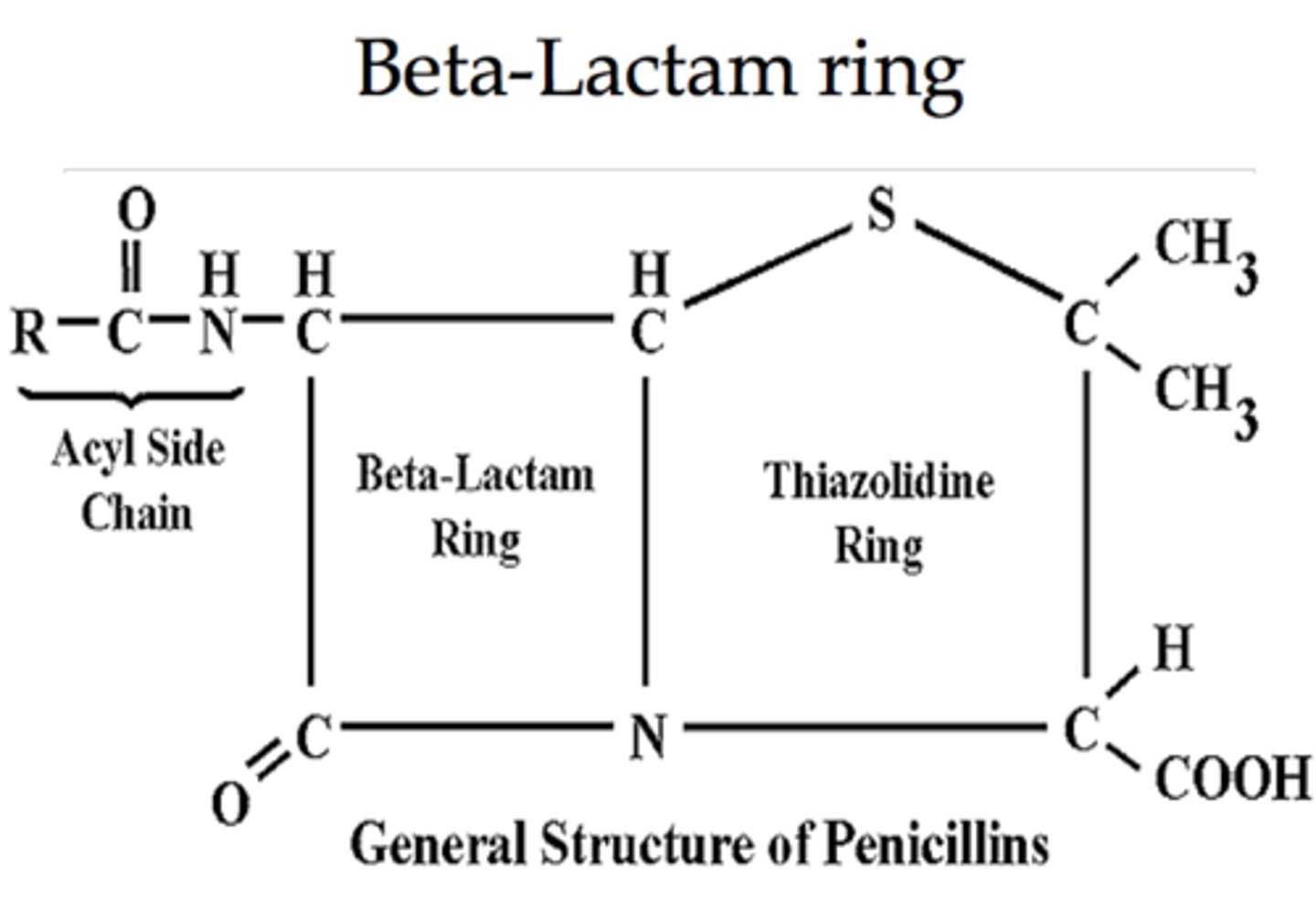
Why is β-lactam more reactive than an amide group?
Because the strained ring system disfavours resonance of nitrogen lone pair.
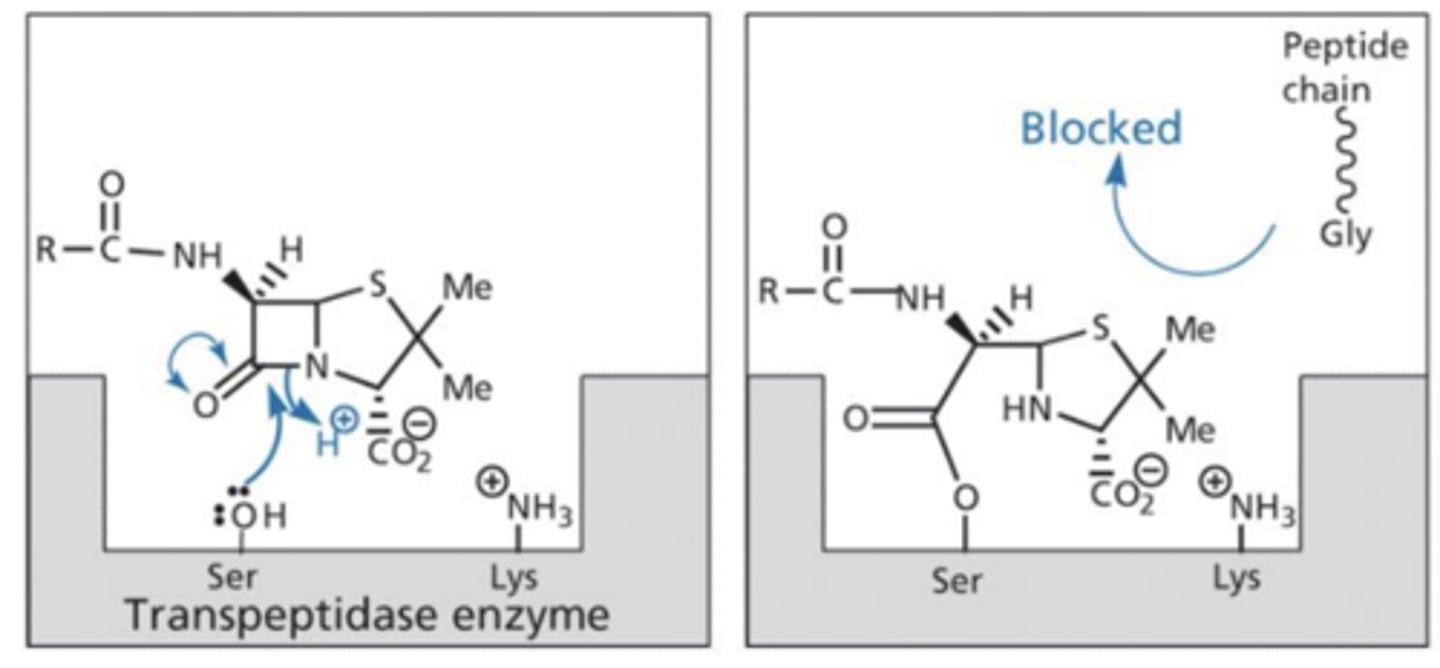
How does the structure of beta-lactam inhibit transpeptidase working?
- The carbonyl on the beta-lactam is attacked by the serine on the transpeptidase enzeyme which breaks the beta-lactam ring causing an ester bond to develop with the serine.
- The carboxylic acid group (at physiolgical pH) forms an Ionic bond with the lysine on the transpeptidase enzyme.
- These 2 interactions prevent the peptide chain on peptidoglycan reaching transpeptidase
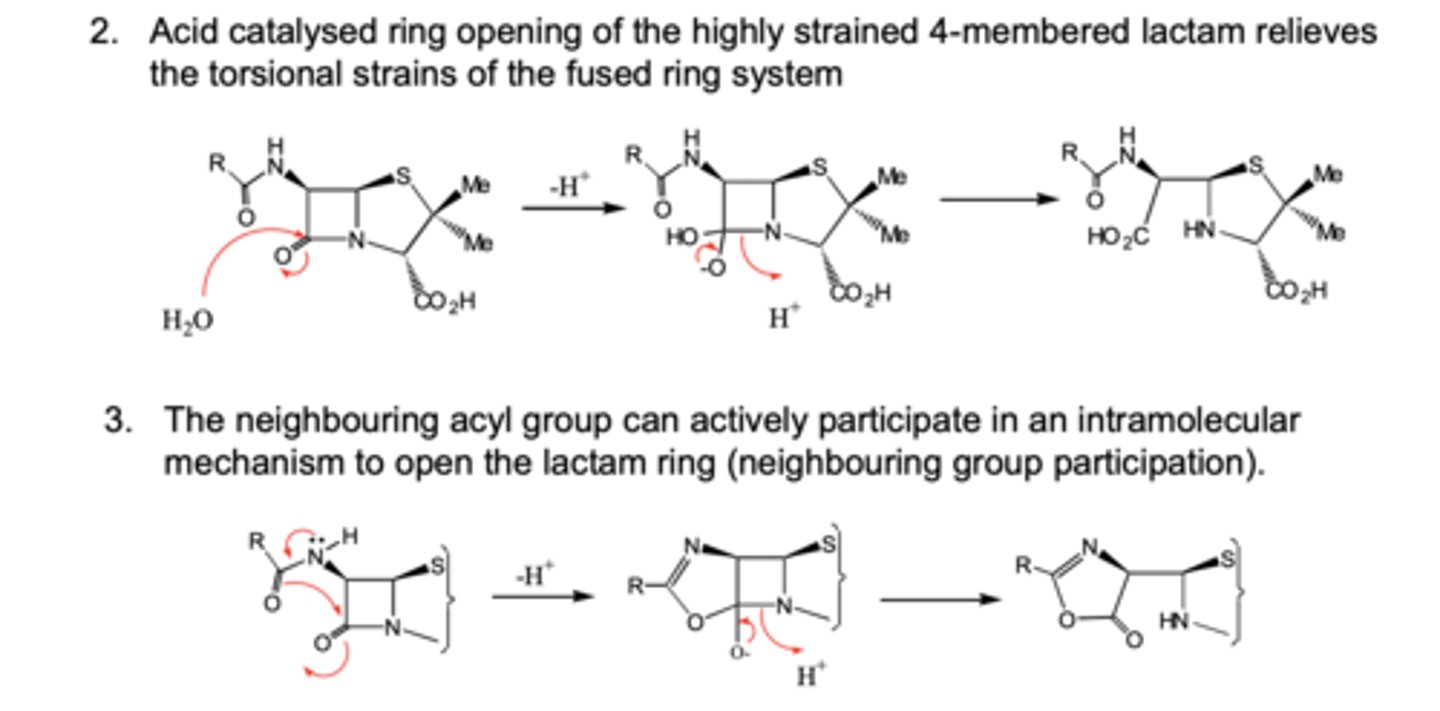
Why is Penicillin G acid sensitive and explain the mechanisms of action?
- Carbonyl in β-lacatam ring is highly susceptible to nucelophilic attack.
- Acid catalysed ring opening of the β-lactam ring relieves torsional strains of the ring system causing breakdown of penicillin.
- Neighbouring acyl groups can actively participate in an intramolecular mechanism to open the lactam ring breaking it down.
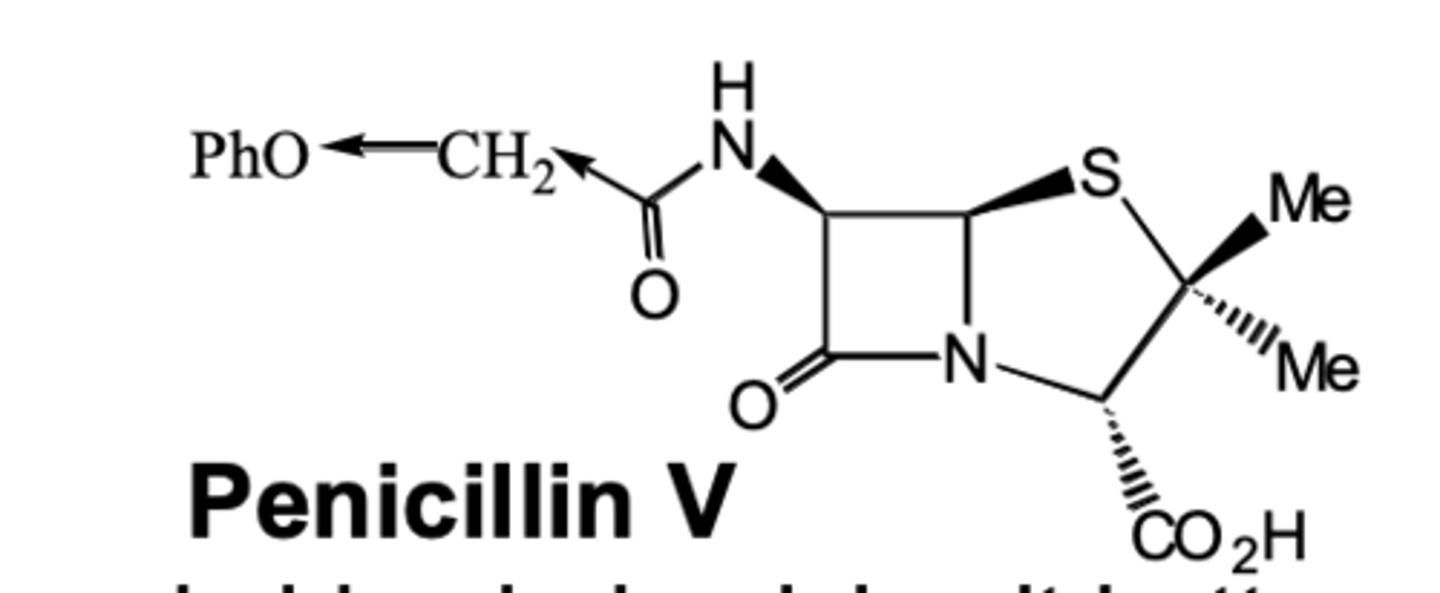
How can acid sensitivity of penicillin be reduced?
Attaching an electron withdrawing group to the carbonyl.
How does attaching an electron withdrawing group reduce acid sensitivity of penicillin?
- Reduces electron density on the carbonyl reducing its tendency to act as a nucelophile prevent neighbouring participation.
- For example, penicillin V has an electronegtaive oxygen on carbonyl increasing acid stability so can be given orally.
How do penicillin resistant bacteria work?
Produce β-lactamase enzymes which catalyse the ring allowing penicillin to undergo acid hydrolysis.
How can penicillin avoid β-lactamase sensitivity of resistant bacteria?
- Use of penicillin analogues which are not recognised by β-lactamase.
- Co-administration of β-lactamase inhibitors.
How does the use of penicillin analogues avoid penicillin resistant bacteria?
- Bulky side chains are used to provide a steric shield towards β-lactamase preventing it from acting on penicillin.
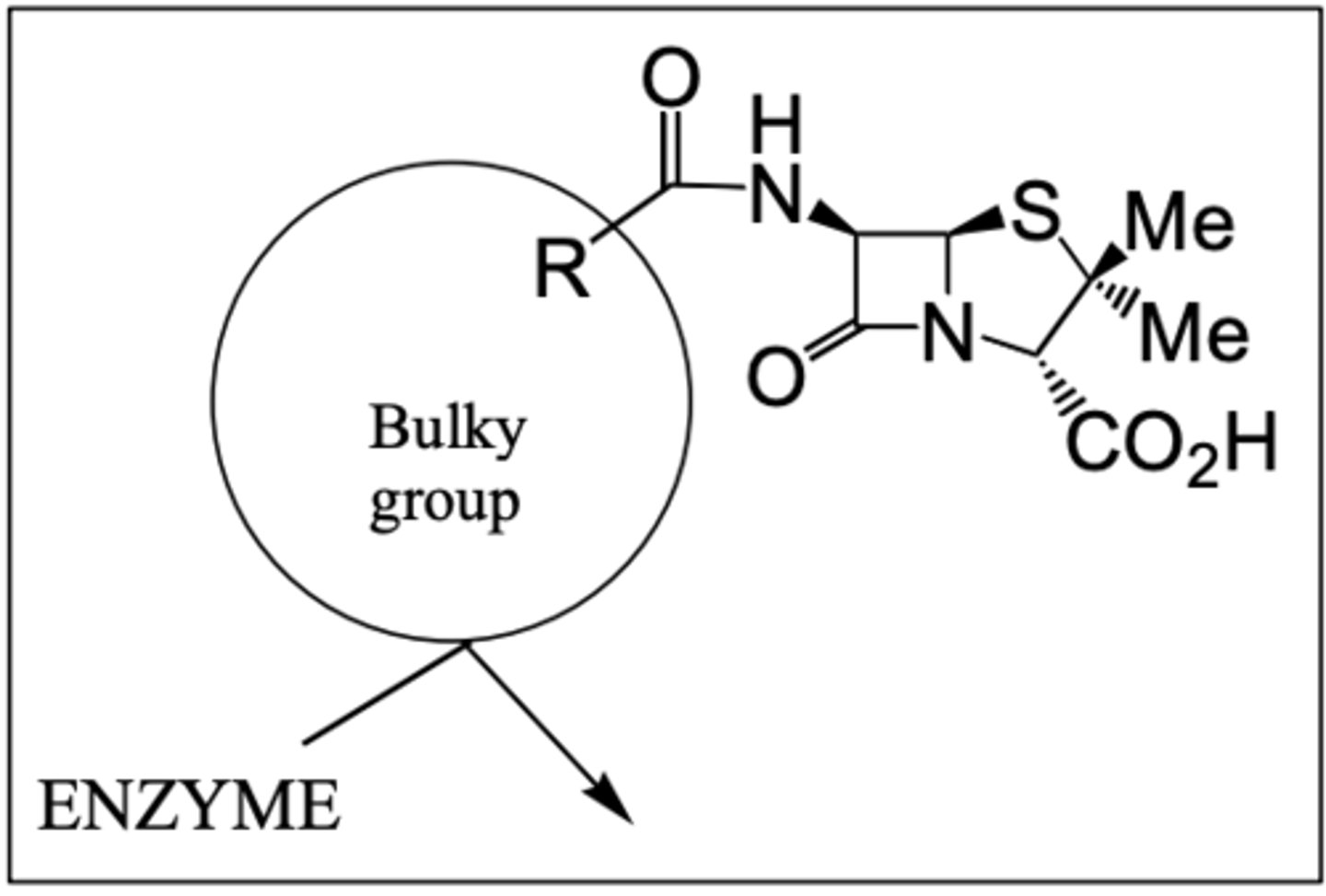
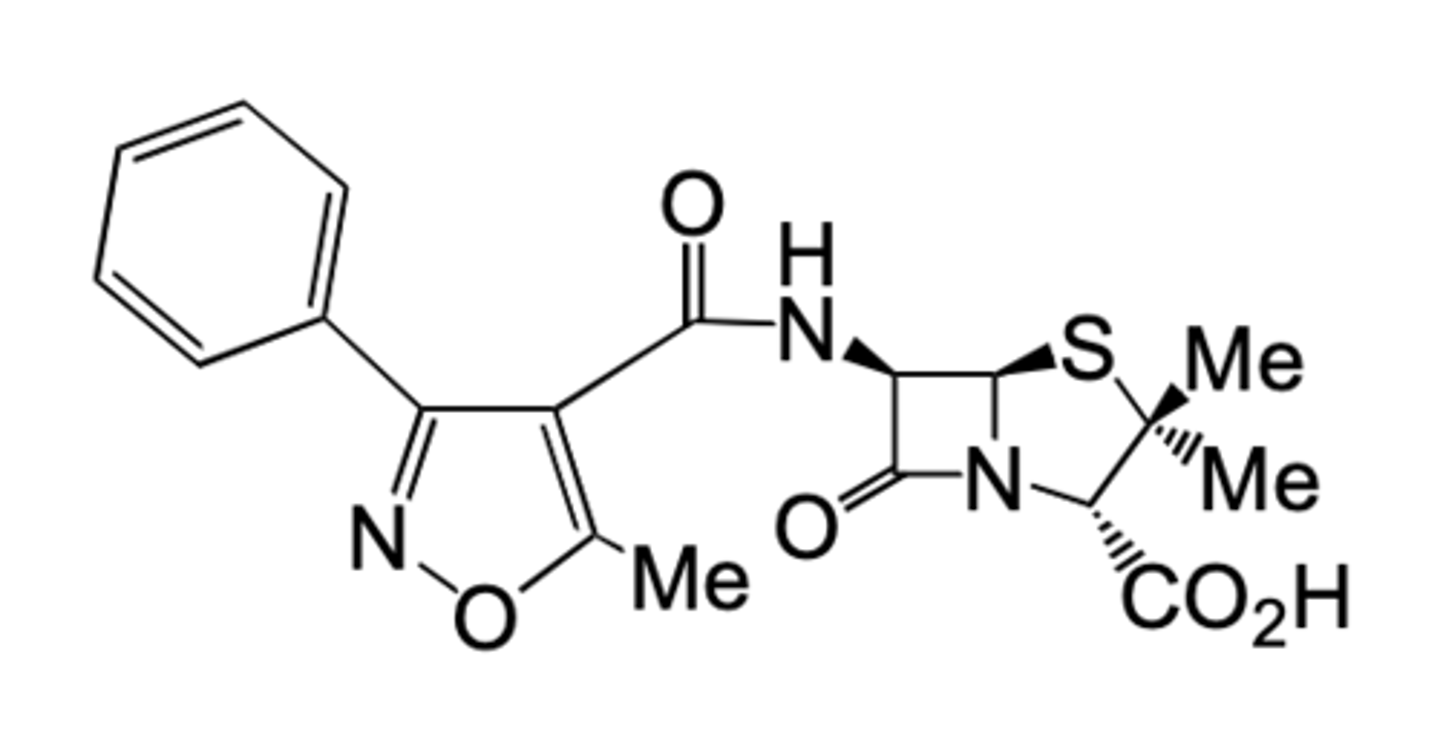
How does oxacillin avoid penicillin resistant bacteria?
Includes a bulku 5-membered ring so resistant to β-lactamases. Also contains an electron withdrawing heterocycle which reduces acid sensitivity so can be taken orally.
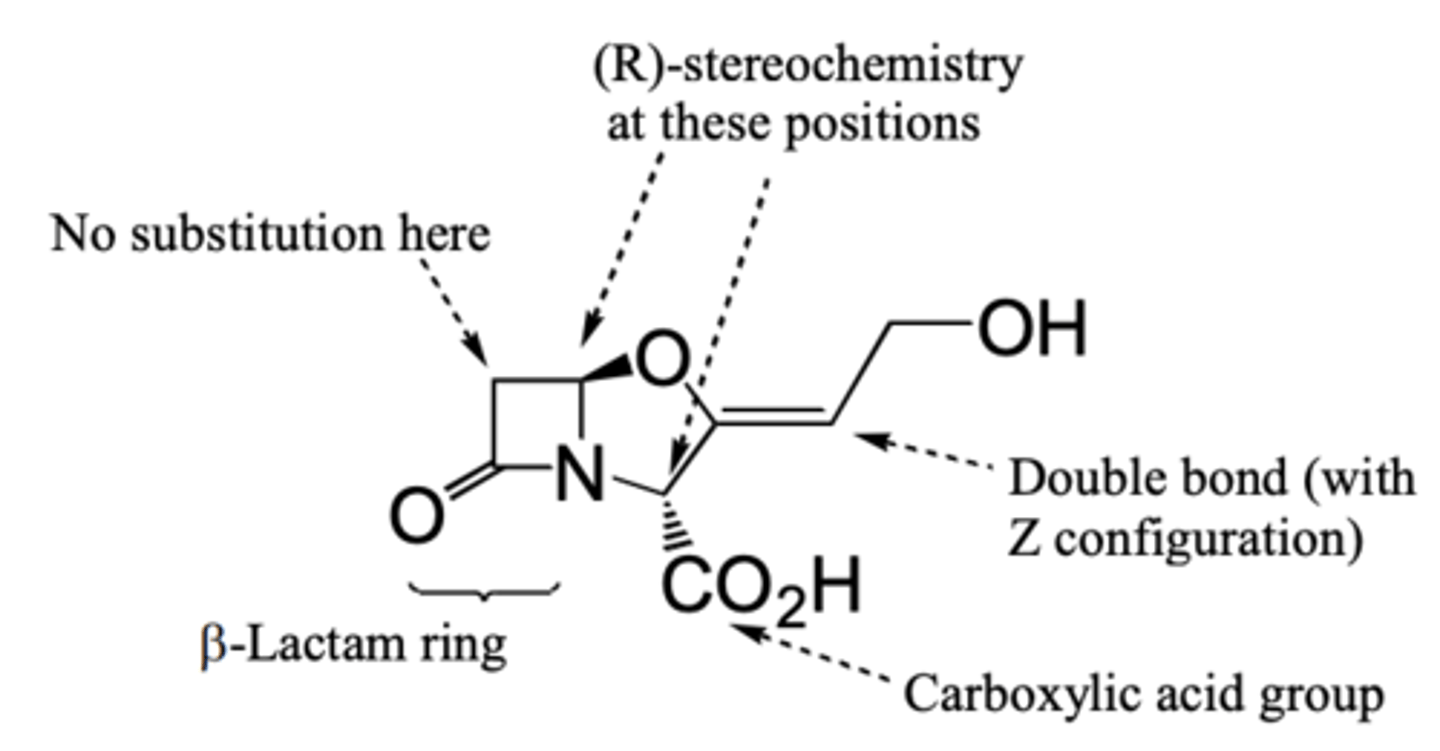
How does co-administration of β-lactamase inhibitors avoid pencillin resistant bacteria?
Administering clavulanic acid (a b-Lactamase inhibitor) in combination with penicillin improves the antibiotic efficacy of penicillin.
What features of clavulanic acid structure is essential for its β-lacatamase inhibition?
Describe the effect broad spectrum penicillin antibiotics on bacteria.
- Showed good activity against G+ve but poor activty against G-ve.
- This is because G-ve have an outer membrane which is impervious to water and hydrophilic molecules like penicillin.
How does penicillin enter G-ve bacteria?
- Enter the periplasmic space of bacteria via porins.
- Transport depends on structure and charge of molecule.
What are porins?
Water filled membrane spanning proteins.
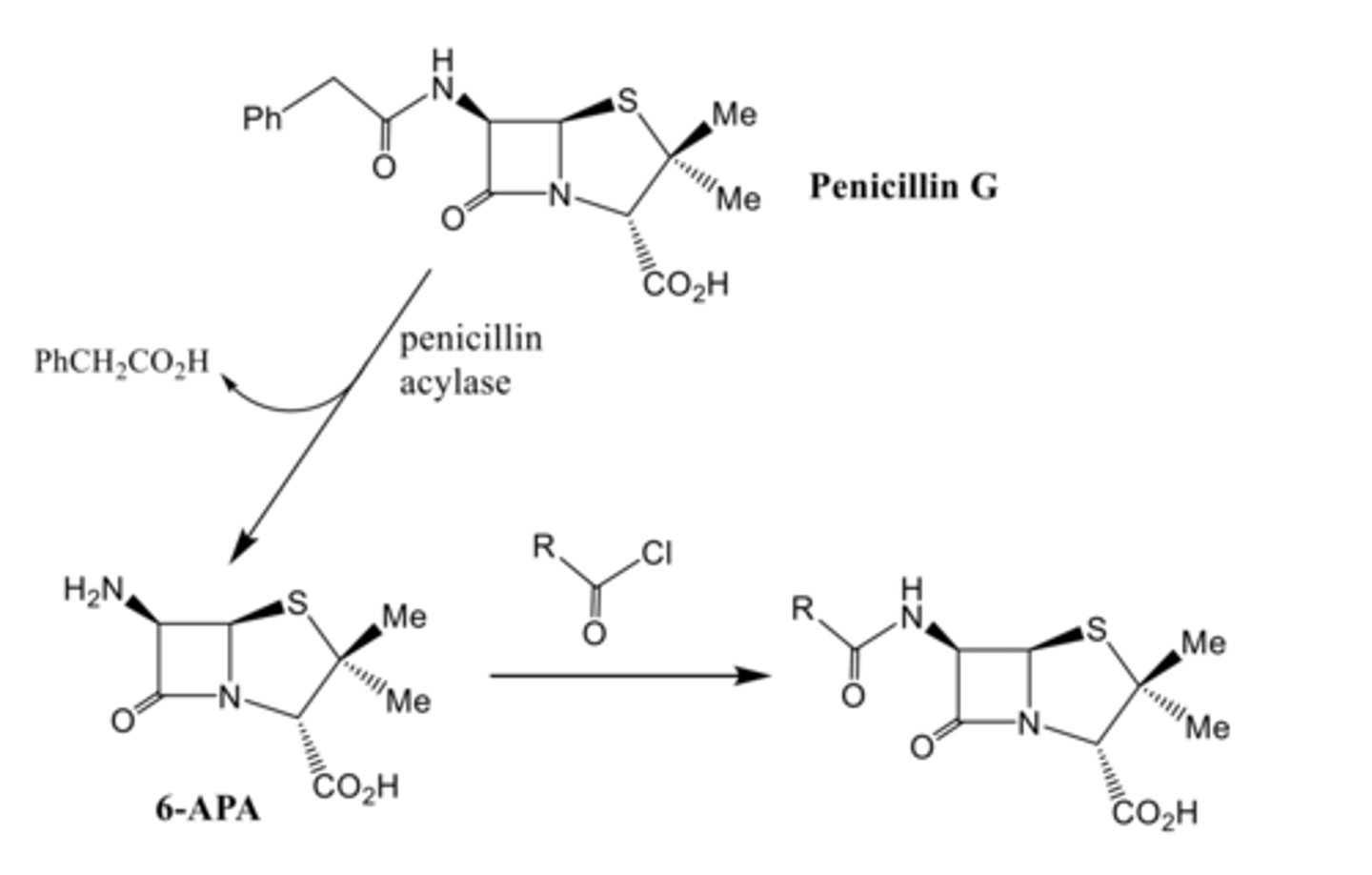
How are penicillin analogues made? Need to know this.
- Semi-synthetically
- Isolate Penicillin G is made first via fermentation and then selectively hydrolyse the side chain using a penicillin acylase enzyme.
- They can then modified through amide bond forming reactions.
What are glycopeptide antibiotic?
- E.g., Vancomycin
- Inhibits peptidoglycan cross linking by binding to the D-Ala-D-Ala terminus.
- Inhibits enzymatic cross-linking by binding to the substrate rather than enzyme.
How do vancomycin resistant bacteria work?
- They have an altered peptidoglycan cross linking process where the terminal D-alanine is replaced by lactic acid.
- This produces an ester bond rather than amide which removes a key H-bonding interaction for vancomycin.