Science chemical unit grade 9
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
Whos Dalton? and draw his diagram.
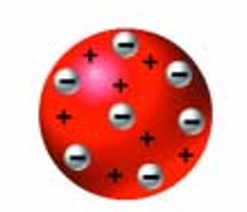
He was the first to define an element as a pure substance that contained no other substances. Gold, oxygen, and chlorine are examples. He put forward the first modern theory of atomic structure. He stated that each element is composed of a particle called an atom. All atoms in a particular element, he said, are identical in mass, and no two elements have atoms of the same mass. For instance, all oxygen atoms have the same mass, which is different from the mass of chlorine atoms. Dalton’s model is sometimes called the “billiard ball model” because he thought of the tiny atoms as solid spheres.

who is J.J. Thomson?. and his model.
Dalton’s work on the structure of the atom was continued by his British physicist student J.J. Thomson. He is credited with being the first person to discover a subatomic particle (a particle smaller than an atom). Thomson, experimenting with cathode rays, concluded that the rays were made up of streams of negatively charged particles. He showed that these particles were much smaller in mass than even a hydrogen atom. He named them electrons.
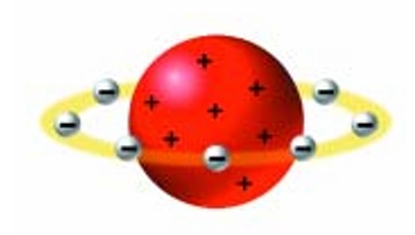
Who is Nagaoka Nagaoka?
Hantaro Nagaoka refined the model of the atom further. In his model, the atom resembled a miniature solar system (Figure 2.15). At the centre of the atom was a large positive charge. The negatively charged electrons orbited around this charge like planets orbiting around the Sun. Most scientists of the day did not agree with this model because existing theories could not explain it.
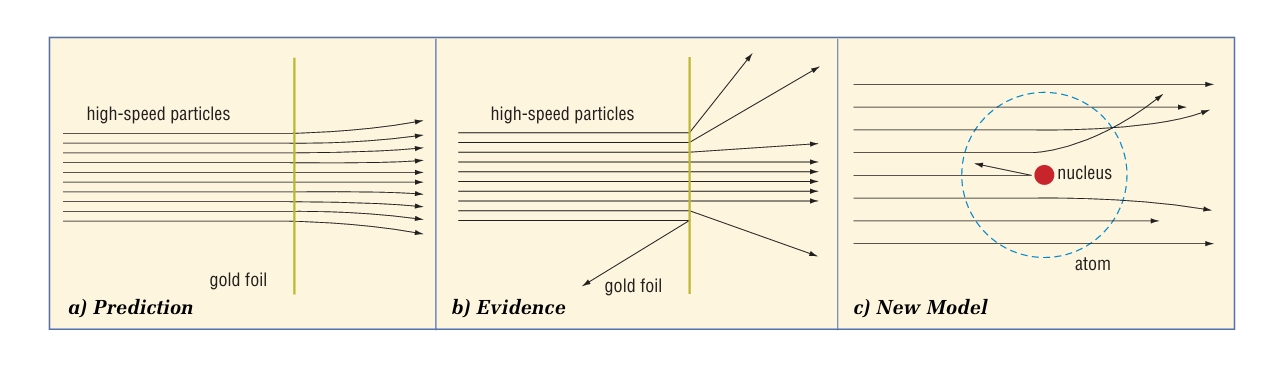
Who is Rutherford? and what is his experiment?
Rutherford conducted experiments in which he shot positively charged particles through thin gold foil. He predicted that all the high-speed particles would pass straight through the foil without being affected by the gold atoms. . Instead, the results showed that while most particles did behave as predicted, some were greatly deflected. Rutherford proposed a new model. He suggested that atoms were mainly empty space through which the positive particles could pass, but at the core was a tiny positively charged centre. (nucleus)He also calculated that the nucleus was only about 1/10 000th the size of the atom—like a green pea in a football field.
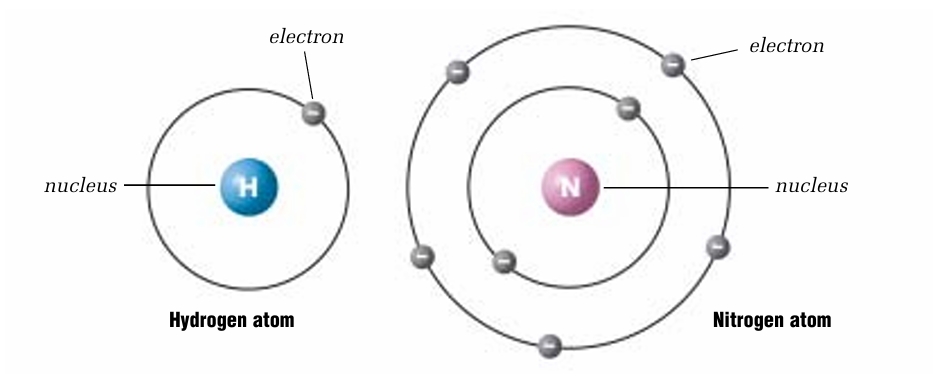
who is Niels Bohr and what are he’s models
Niels Bohr who, working with Rutherford, suggested that electrons do not orbit randomly in an atom. Bohr said that they move in specific circular orbits, or electron shells,he believed that electrons jump between these shells by gaining or losing energy. For his work in studying the atom,
German alchemist Andreas Libau
Andreas Libau published Alchemia, a book describing the achievements of alchemists. In it, however, Libau also explained how to prepare chemicals such as hydrochloric acid. This type of information made his book the first chemistry text ever printed
Robert Boyle
Robert Boyle experimented with the behaviour of gases. He was interested in what happened when gases were placed under pressure.Through his experiments and observations, Boyle became convinced that matter was made up of tiny particles, just as Democritus.Boyle believed that the tiny particles, existing in various shapes and sizes, would group together in different ways to form individual substances. Boyle felt that the purpose of chemistry was to determine the types of particles making up each substance.
Democritus
He observed that a rock could be broken into smaller and smaller pieces.In about 400 .C., the Greek philosopher Democritus used the word atomos to describe the smallest particles that could not be broken further. Atomos means “indivisible.Democritus stated that each type of material was made up of a different type of atomos. These different particles, he believed, gave each material its own unique set of properties. By mixing different atomos, you could make new materials with their own unique properties.
Greek philosopher, Aristotle,
. Alchemists
Alchemists believed that it should be possible to change metals into gold. They were not interested in understanding the nature of matter.Even though they weren’t real scientists, alchemists performed some of the first chemistry experiments. In doing so, they invented many useful tools that we still use in labs today,(an activity that is not a real science because it includes the use of magic).
Antoine Laurent Lavoisier
the French scientist Antoine Laurent Lavoisier studied chemical interactions. He had developed a system for naming chemicals. for now all scientists could use the same words to describe their observations. That made it easier to compare the results of their experiments. Using his naming system, Lavoisier defined some of the substances discovered to that time, including hydrogen, oxygen, and carbon. Lavoisier is called the “father of modern chemistry.
Atomic mass
sis the mass of one atom of an element.Atomic mass is measured by atomic massunit(amu).