Lewis stuctures and Lewis acids and bases
0.0(0)
Card Sorting
1/20
Earn XP
Description and Tags
Last updated 2:23 PM on 1/23/23
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
1
New cards
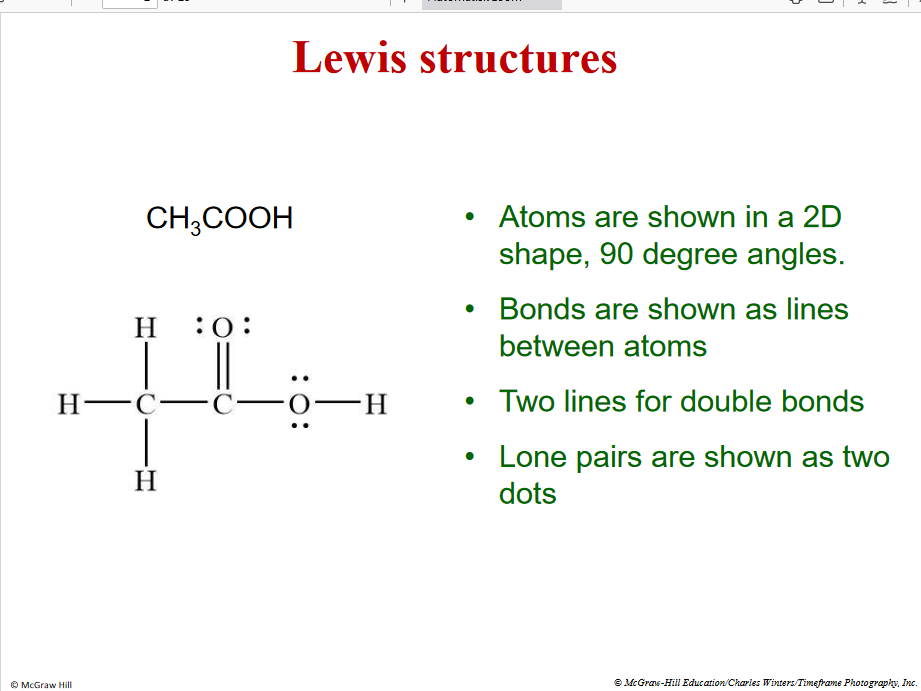
Lewis stuctures
90 degree angle
8 valence electron except H - Oxtate rule for early p block element
Double bond = 4 electrons
Single bond = 2 electrons
8 valence electron except H - Oxtate rule for early p block element
Double bond = 4 electrons
Single bond = 2 electrons
2
New cards
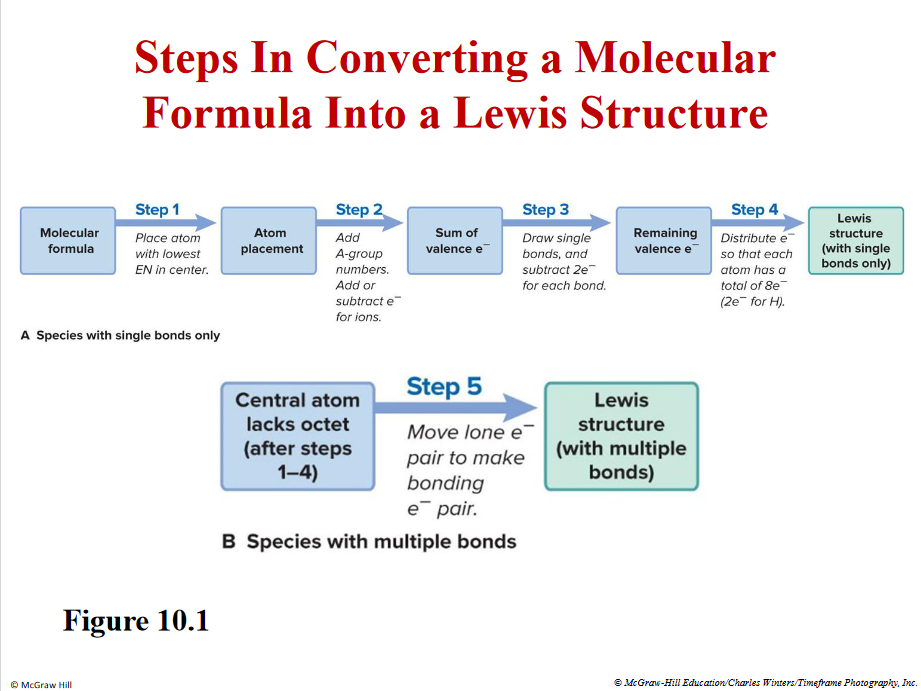
Molecule → lewis structure
Remember the one atom with lowest EN in the middle except H - can’t make more than 1 bond)
If lacking the octett rule → forms double bonds
If lacking the octett rule → forms double bonds
3
New cards
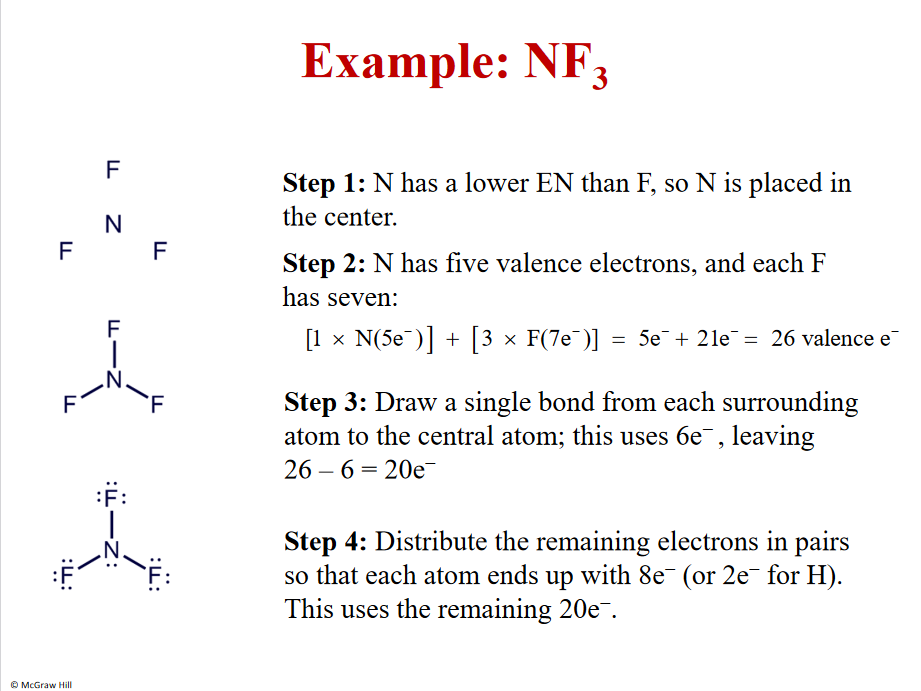
Example NF3
4
New cards
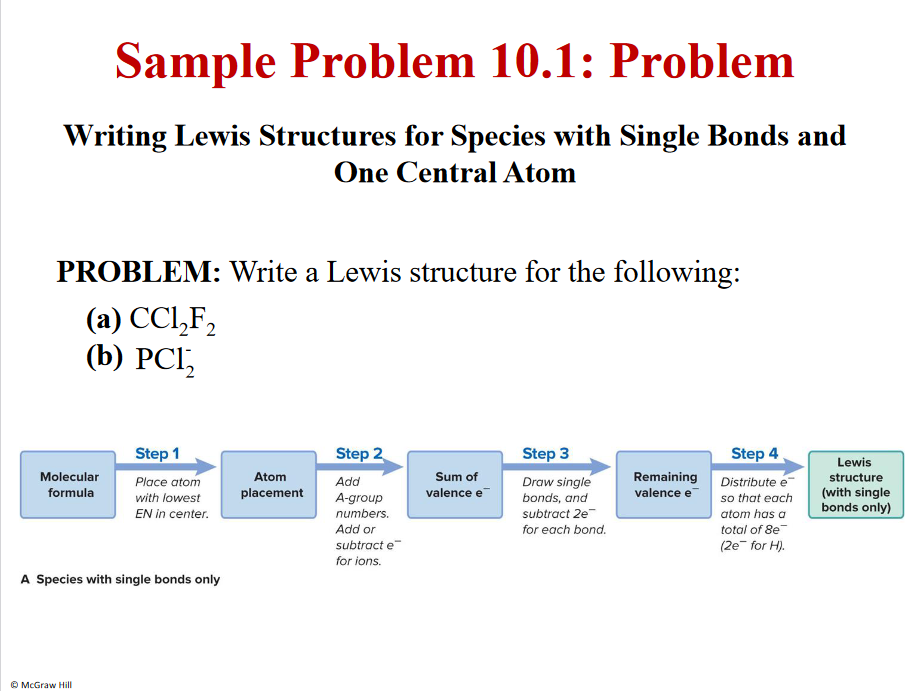
Problem
C - lowest EN in centere
Cl,F, H - NOT IN CENTER - ONLY 1 BOND
Cl,F, H - NOT IN CENTER - ONLY 1 BOND
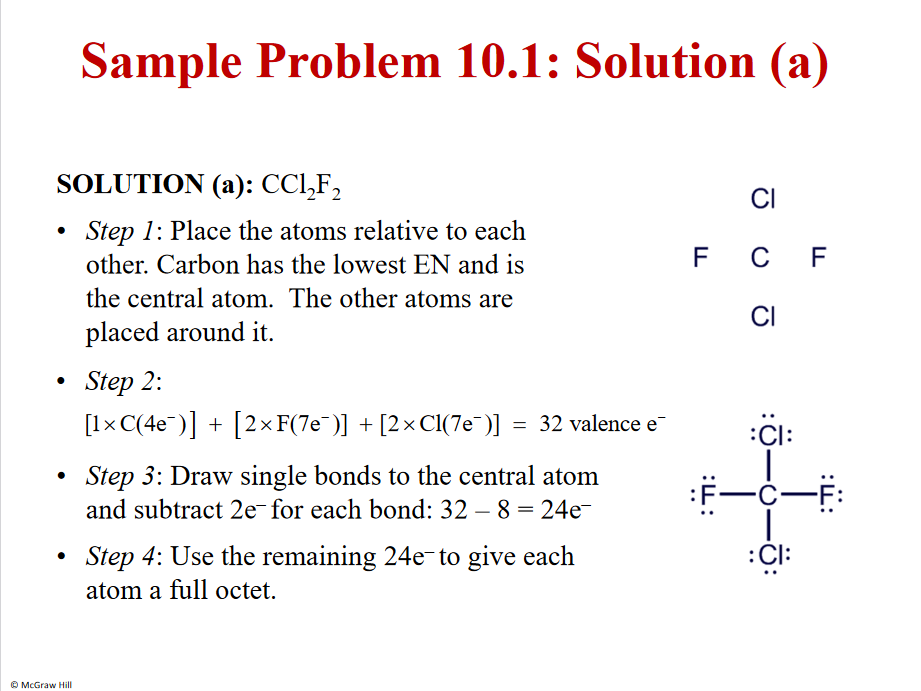
5
New cards
Solution b
Remember!! Add - or + - adding or taking away an e
Dosen’t matter were you put the extra electron
Dosen’t matter were you put the extra electron
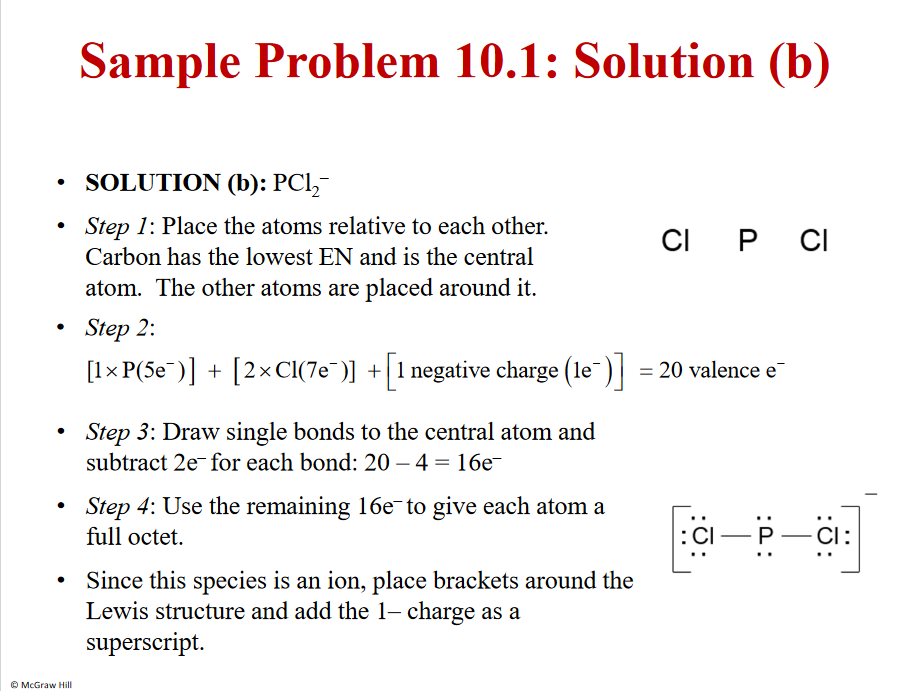
6
New cards
Methanol, n2, c02
N---N - Octett rule
7
New cards
Resonance structures
Both structures are correct
There are multiple ways to draw them
Equal strength in both bonds
However - if it would be 1 double bond and one single bond the double bond would be much shorter + stronger than the single bond. But the molecule is equal in reality - thats why it has 2 resonance structures - BOTH correct, unclear were the bond is
There are multiple ways to draw them
Equal strength in both bonds
However - if it would be 1 double bond and one single bond the double bond would be much shorter + stronger than the single bond. But the molecule is equal in reality - thats why it has 2 resonance structures - BOTH correct, unclear were the bond is
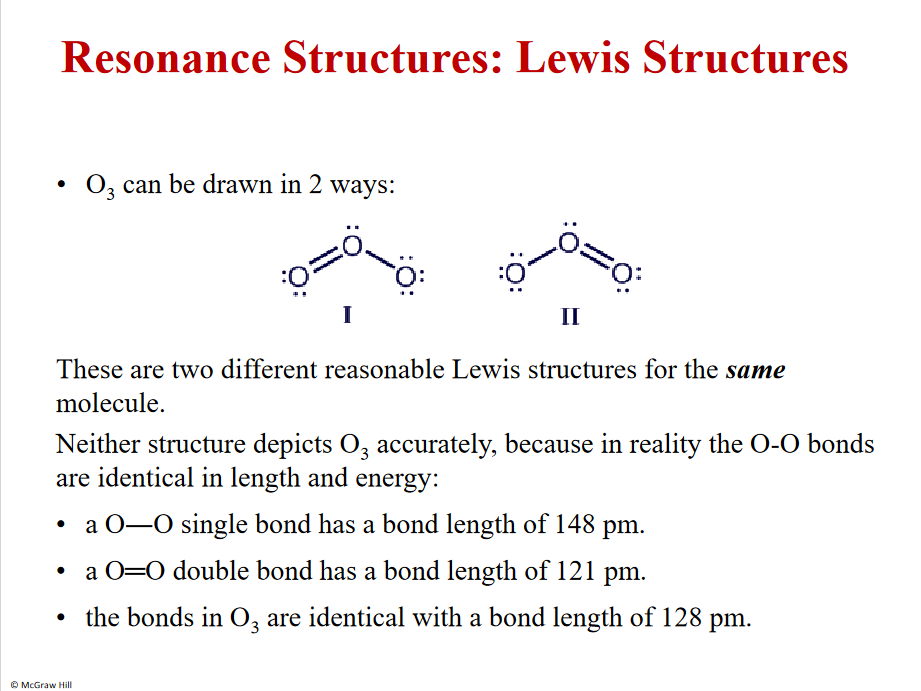
8
New cards
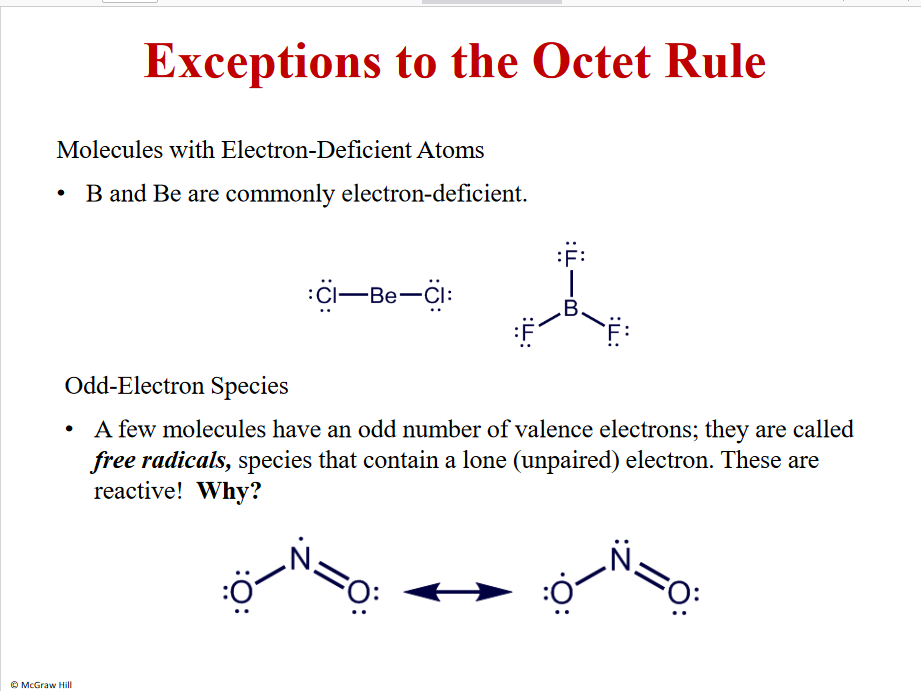
Exceptions to the octet rule
B and Be
Free radicals - very reactive- more than 8 e - expanded shell
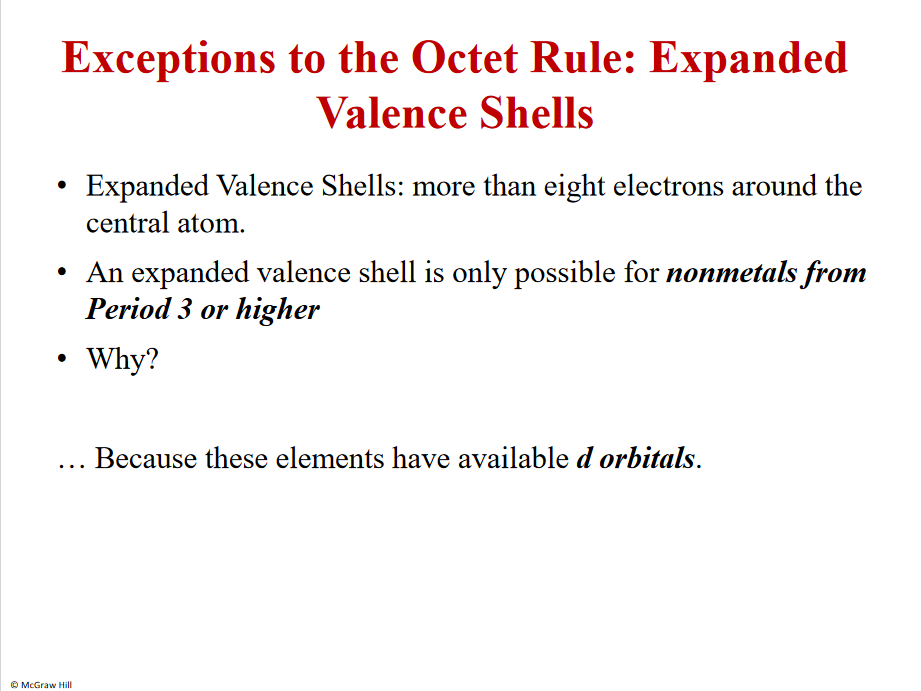
NONmetalls - period 3 and higher - due to 3d orbital exists - expanded
(s+3p - 8)
Free radicals - very reactive- more than 8 e - expanded shell
NONmetalls - period 3 and higher - due to 3d orbital exists - expanded
(s+3p - 8)
9
New cards
Expanded valence shells
10
New cards
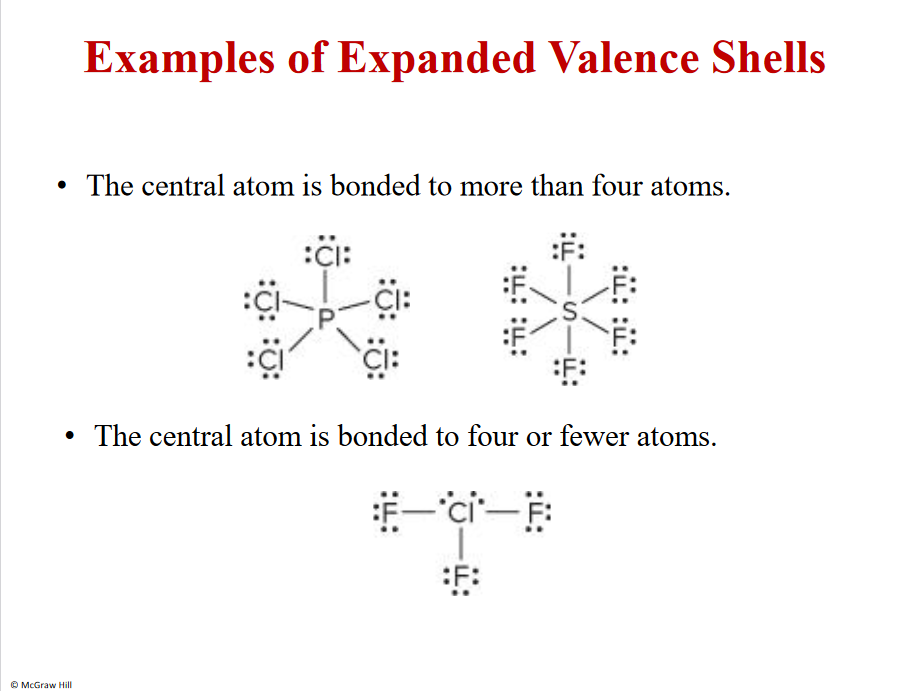
Examples of expanded valence shells
Bonding looses energy - the goal
11
New cards
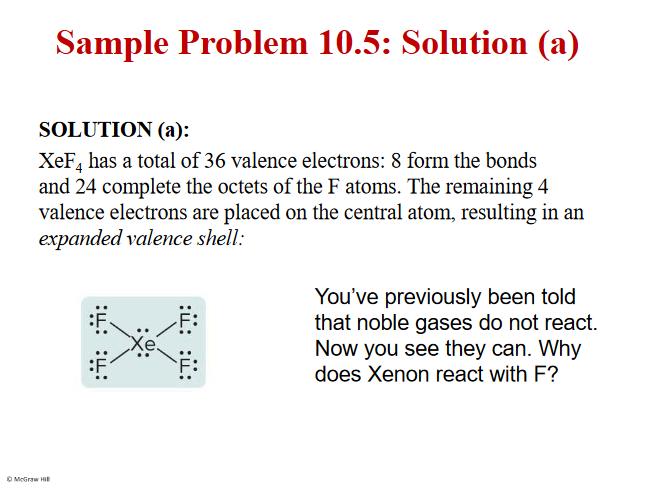
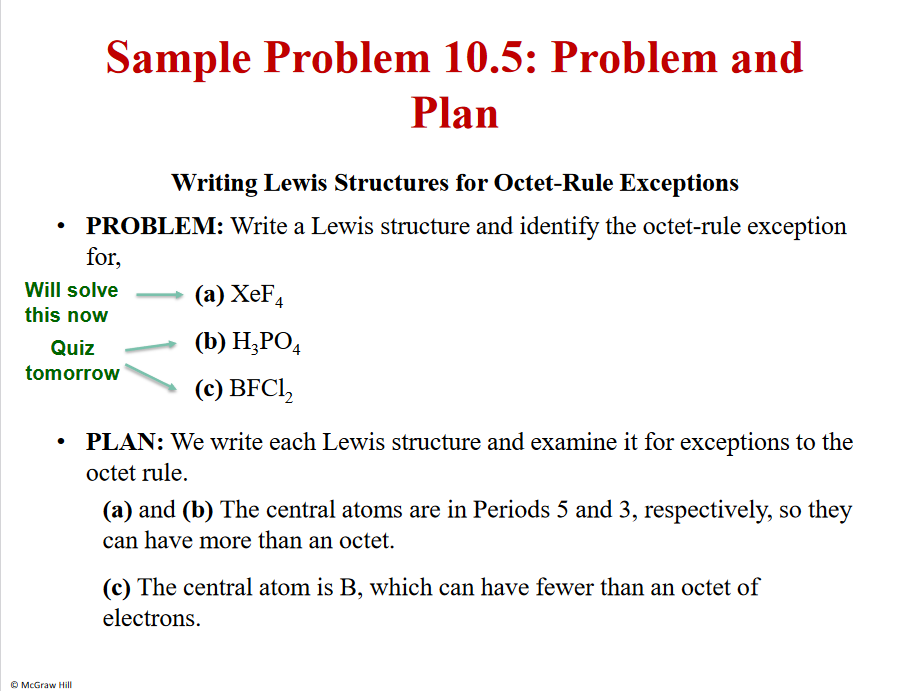
Problem and plan
2 extra lone pairs
F is very EN- many protons- dragging the electron away from the Xe
Lower energy
F is very EN- many protons- dragging the electron away from the Xe
Lower energy
12
New cards
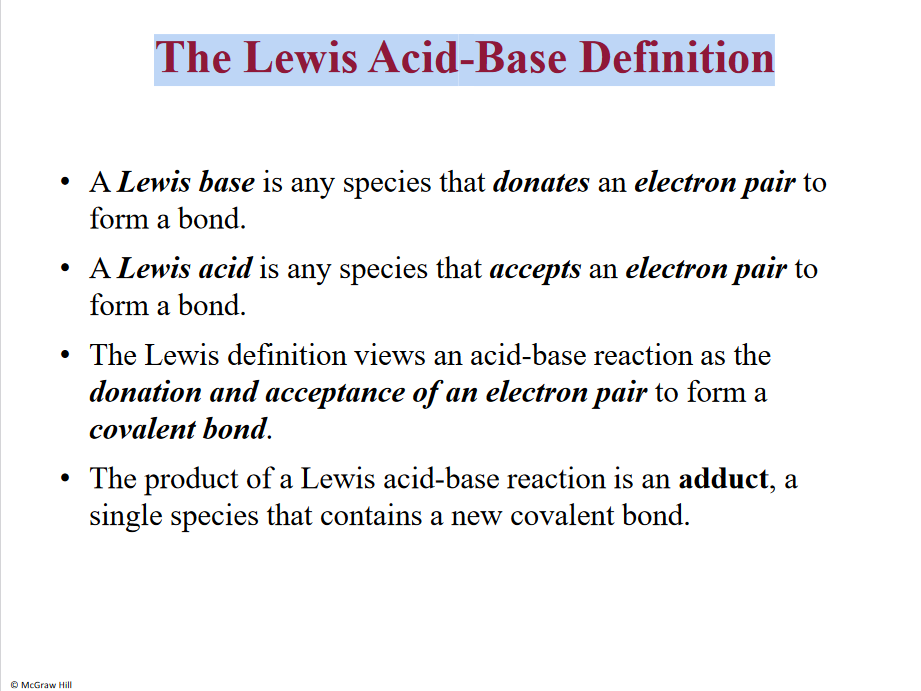
The Lewis Acid-Base Definition
Lewis base/acid not the same as Bronstedt
H+ - Acid - anything accepts an electron pair
Lewis base - donates an electron pair
H20 is a base- dative bond
H+ - Acid - anything accepts an electron pair
Lewis base - donates an electron pair
H20 is a base- dative bond
13
New cards
Lewis Acids and Bases
Remember lone pairs!!
Vacant orbital- EMPTY
Vacant orbital- EMPTY

14
New cards
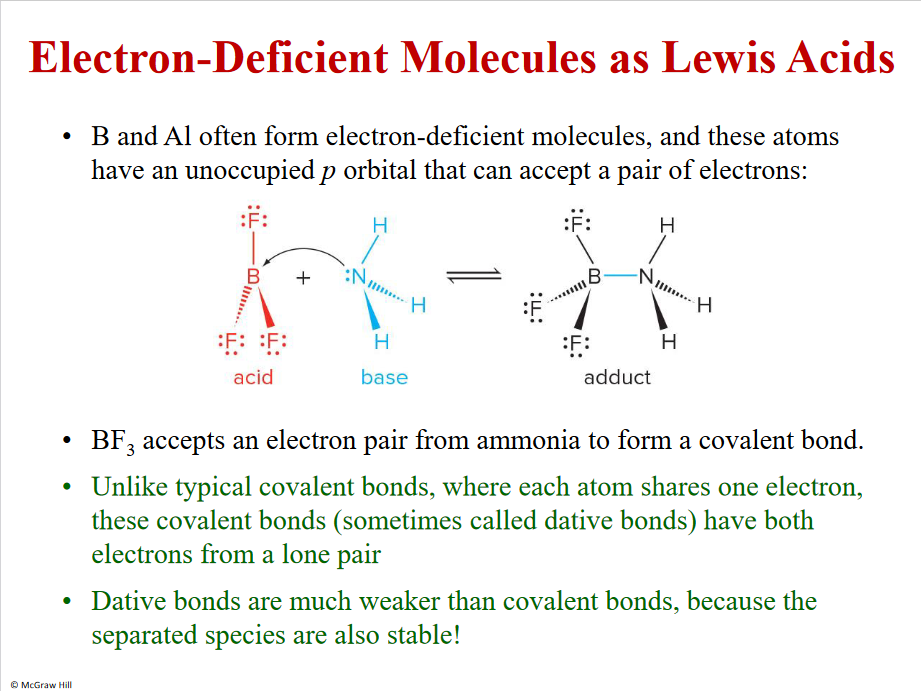
Electron-Deficient Molecules as Lewis Acids
Dative bond - weaker than a covalent bond, easily broken
15
New cards
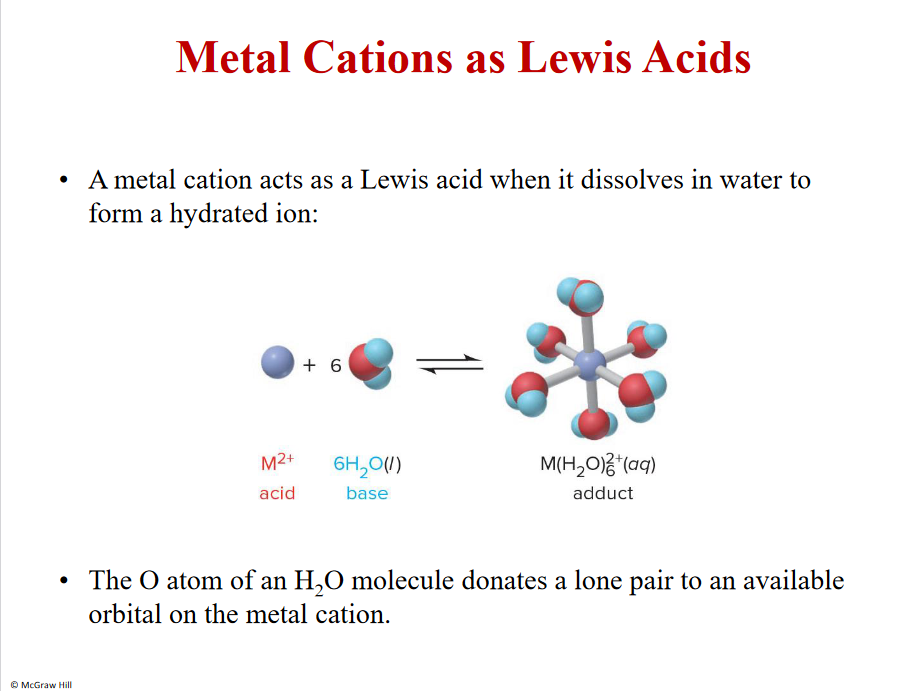
Metal Cations as Lewis Acids
Metals- lewis acids- forms dative bond- hemoglobin, chlorophyll
EMPTY ORBITALS
EMPTY ORBITALS
16
New cards
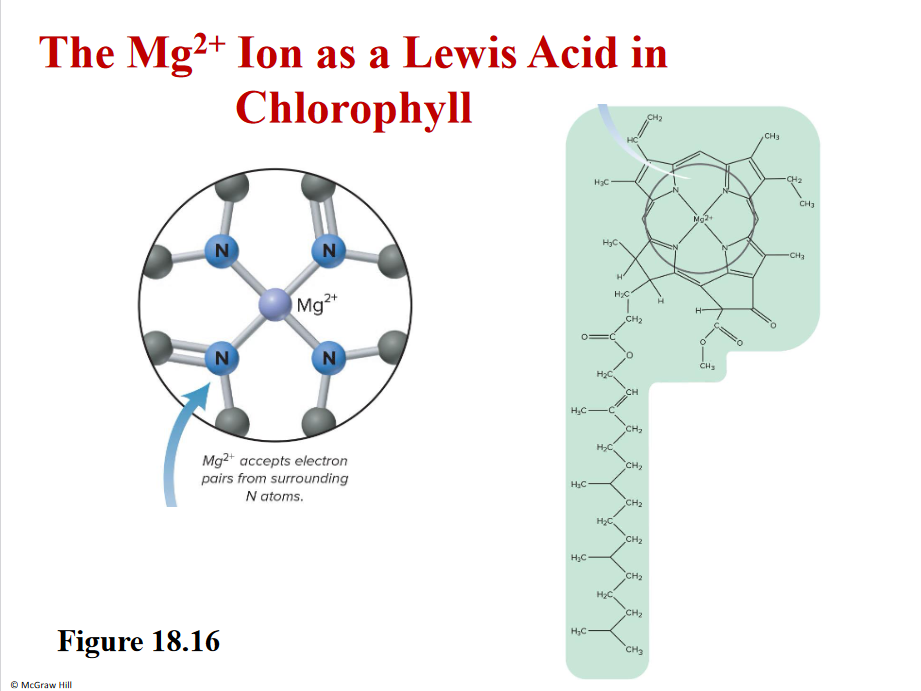
Chlorophyll
Mg2+= lewis acid
EMPTY ORBITALS
Lewis base have LONE PAIRS
EMPTY ORBITALS
Lewis base have LONE PAIRS
17
New cards
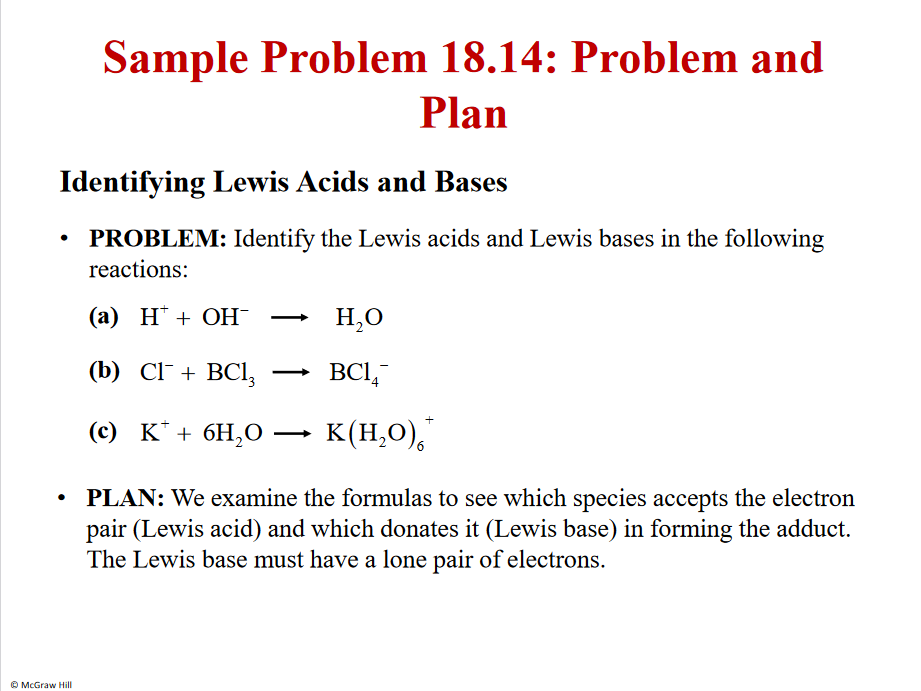
Problem
18
New cards
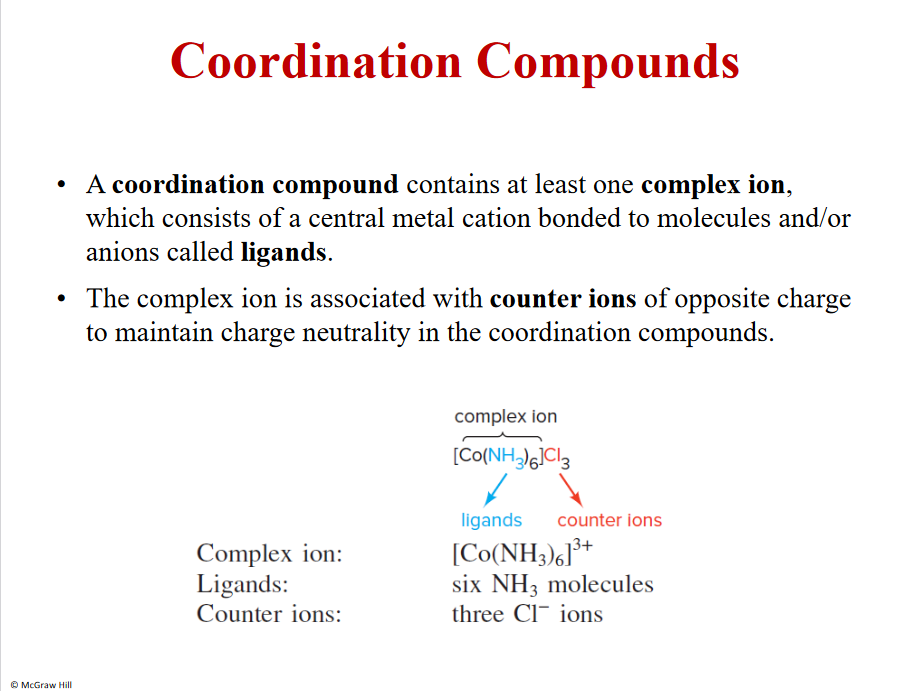
Coordination Compounds
Ligands- lewis bases, around metals
Counter ions- outside the klammrar/box
Complex ion- metal cation bonded to ligands
Counter ions- outside the klammrar/box
Complex ion- metal cation bonded to ligands
19
New cards
Coordination Number
Specific for each element
Number of ligands bonded directly to the metal ion
Number of ligands bonded directly to the metal ion

20
New cards

Ligands and examples
NH3- ions or molecules, lewis bases
Mg2+- lewis aid in chlorophyll
Acids are often metals, compounds or H+- THEY HAVE EMPTY ORBITALS
Mg2+- lewis aid in chlorophyll
Acids are often metals, compounds or H+- THEY HAVE EMPTY ORBITALS
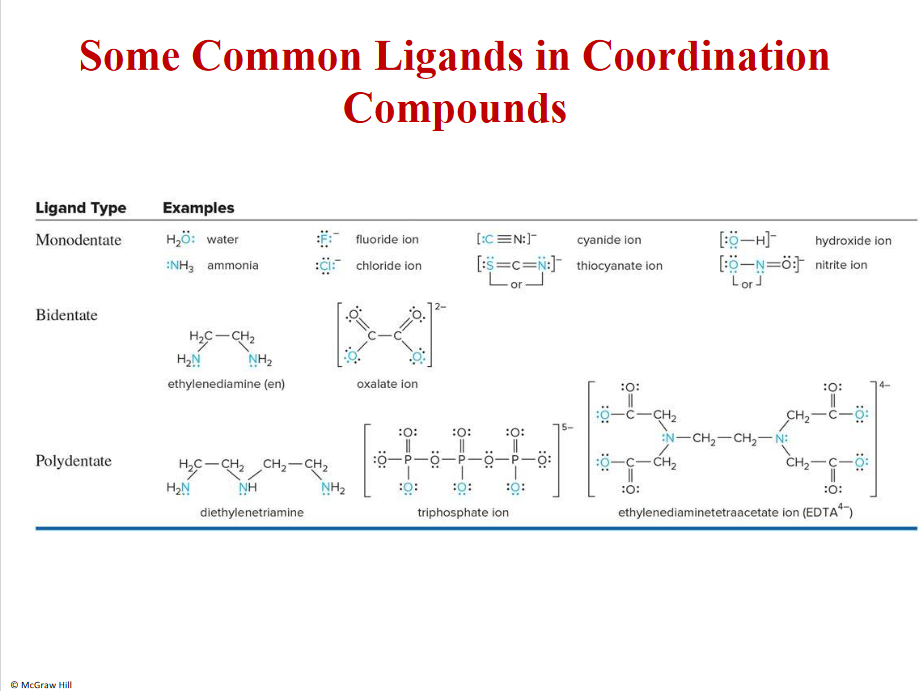
21
New cards
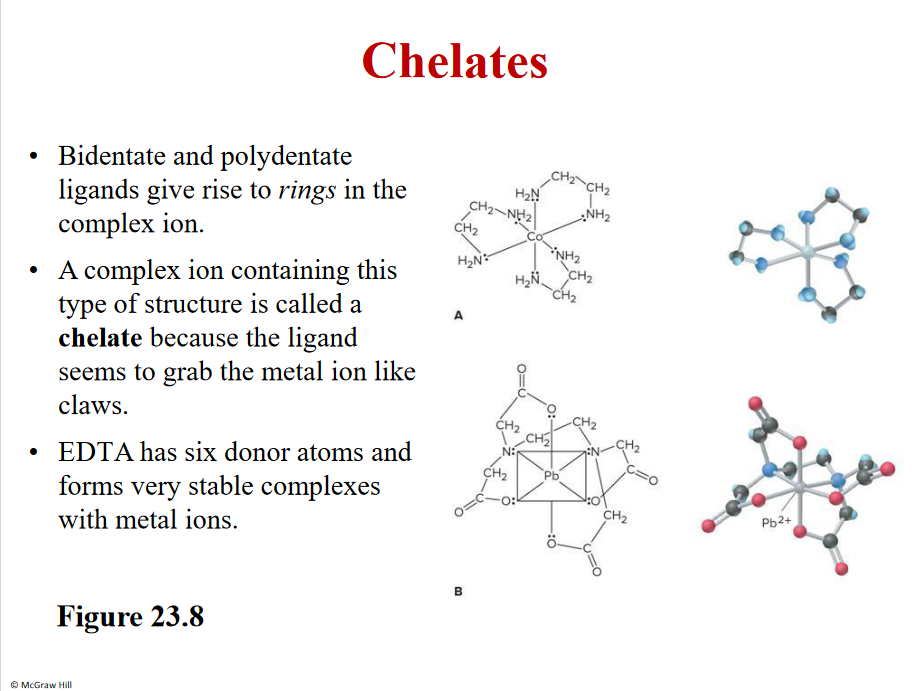
Chelates
Form rings
Bi- two bonds
Poly- more than 2
Mono- 1 bond
Grabbing a metal ion to neautralize
Severeal lon pairs - more bonds
Bi- two bonds
Poly- more than 2
Mono- 1 bond
Grabbing a metal ion to neautralize
Severeal lon pairs - more bonds