Energy changes
1/29
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
What are the two types of energy changes ?
Exothermic
Endothermic
What is an Exothermic reaction and state examples ?
A reactionf that transfers energy to its surrounding
The temperature of the surroundings increase
Examples include
Combustion
Oxidation
Neutralisation
What are the everyday uses of exothermic reactions ?
Self-heating cans
Hand warmers
What is an endothermic reaction and what are some examples?
A reaction that takes in energy from the surroundings
The temperature of the surroundings decreases
Examples include:
Thermal decomposition
The reaction of citric acid and sodium hydrogencarbonate
What are the everyday uses of endothermic reactions?
Some sports injury packs are based on endothermic reaction
What does the energy profile of an exothermic reaction look like?
• Products have lower energy than reactants
• Energy change for an exothermic reaction is negative
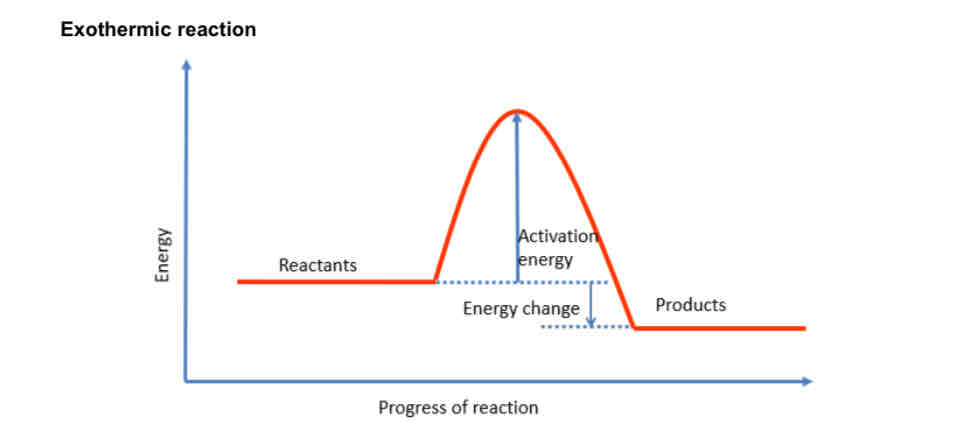
What is the activation energy ?
The minimum amount of energy that particles must have to react is called the activation energy
How do we know a reaction is an exothermic reaction of the overall energy change is a negative number?
This is an exothermic reaction because the energy released from forming new bonds
is greater than the energy needed to break existing bonds.
How do we know a reaction is an endothermic reaction of the overall energy change is a postive number?
This is an endothermic reaction as the energy needed to break existing bonds is
greater than the energy released from forming new bonds.
How do cells produce chemicals?
Cells contain chemicals which react to produce electricity
How can a simple cell be made?
• A simple cell can be made by connecting two different metals in contact with an electrolyte.
• The two electrodes are metals as they conduct electricity delocalised electrons which carry electrical charge.
• The electrolyte is a solution that conducts electricity and contains ions which are free to move and carry electrical charge.
How can batteries produce a greater voltage?
Two or more cells connected in series produce a larger voltage
What is the voltage produced by the cell dependent on?
Type of electrode
Type of electrolyte
In non rechargeable batteries when does it stop working?
When one of the reactants been used up
Are alkaline batteries rechargeable or non rechargeable?
Non rechargeable
If there is a larger difference in reactivity, what does this tell us about the voltage ?
Greater voltage
What type of ions do alkaline batteries include and what are the uses of this battery?
OH- ions (hydroxide ions)
Used in remote controls
Why can rechargeable batteries recharge?
Rechargeable cells and batteries can be recharged because the chemical reactions are reversed when an external electrical current is supplied.
What are the difference between the processes in electrolysis and in a chemical cell?
o Electrolysis uses electricity to produce a chemical reaction
o Cells use a chemical reaction to produce electricity
When will the voltage be zero in a cell?
• The electrodes are made of the same metal
• The reactivity of the metals is the same
What is a fuel cell?
Fuel cells are supplied by an external source of fuel (eg hydrogen) and oxygen or air.
The fuel is oxidised electrochemically within the fuel cell to produce a potential difference.
What does the overall reaction in a hydrogen fuel cell involve ?
• The overall reaction in a hydrogen fuel cell involves the oxidation of hydrogen to produce water.
How do fuel cells produce electricity?
Fuel and oxygen to produce electrical energy
How do hydrogen-oxygen fuel cells work?
• Electrolyte is often a solution of phosphoric acid and the electrodes are often porous carbon with a catalyst
• Hydrogen gas goes into the anode and oxygen into the cathode
• At the anode the hydrogen loses electrons to form hydrogen ions
• Hydrogen ions in the electrolyte moves to the cathode
• At the cathode, oxygen gains electrons from the cathode and reacts with hydrogen ions to make water
• The electrons flow through an external circuit from the anode to the cathode. This is the electric current.
What are the products of a hydrogen-oxygen fuel cell?
Water and energy
As hydrogen-oxygen fuel cells involve a redox reaction what occurs at the anode and cathode + half equations?
Anode - Oxidation
Hydrogen loses electrons to form hydrogen ions
H2 → 2H+ + 2e-
Cathode - Reduction
Oxygen gains electrons from the cathode and reacts with hydrogen ions to make water
O2 + 4H+ 4e- → 2H2O
What is the overall equation for a hydrogen-oxygen fuel cell?

What are the advantages and disadvantages of Hydrogen fuel cells?
Hydrogen Fuel cells
Advantages
• No pollutants-Produces only water
• Do not need recharging
• Hydrogen can be made renewable if made by electrolysis using renewable energy.
Disadvantages
• Hydrogen is highly flammable
• Hydrogen is difficult to store/takes up a lot of space
• Hydrogen not renewable if produced using fossil fuels
• Not many hydrogen filling stations
What are the advantages and disadvantages of rechargeable batteries?
Rechargeable batteries
Advantages
• Charging points more widely available
• Rechargable
Disadvantages
• May release toxic chemicals on disposal
• Rechargeable batteries have finite life-time
• Can catch fire
• Store less energy than fuel cells
What is a potential alternative to rechargeable batteries and cells?
Hydrogen fuel cells