practicals - 7a + 7b - needs finishing!!
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
What needs to be done to measure a reaction rate?
The concentration of one of the reactants / products must be measured over a period of time
Temperature must be kept constant as rate varies with temperature
What are the two ways to measure the rate of a chemical reaction?
Using a continuous monitoring method (7b)
Using an initial rates method (7a)
The method chosen depends on..
the substance whose concentration is being measured + speed of the reaction
Give the method for continuous monitoring
You would measure either the ‘amount’ of a product formed or the reactant lost continuously throughout the course of one reaction
Therefore, could measure concentration of a species reacted / formed, volume of gas formed or mass lost during a reaction
Plot a concentration against time, volume against time or mass against time → deduce the rate + order of reaction
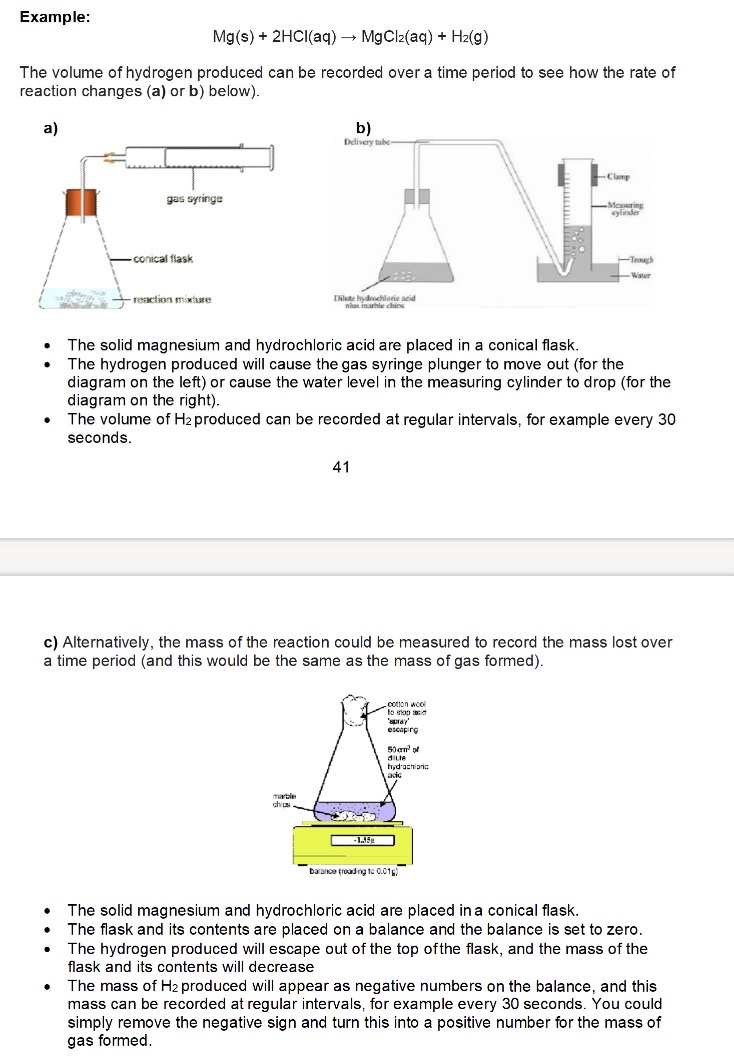
Even though the units of rate are normally mol dm-3s-1, why can we still use volume / mass of gas formed?
These are proportional to concentration → the units will just be different
How to find the rate of reaction from concentration / volume / mass over time?
If a graph of the concentration, volume of gas or the mass of gas produced is plotted against time, rate can be found by calculating the gradient
Collect data + plot graph
Draw a line of best fit
Draw tangent
Find gradient of tangent
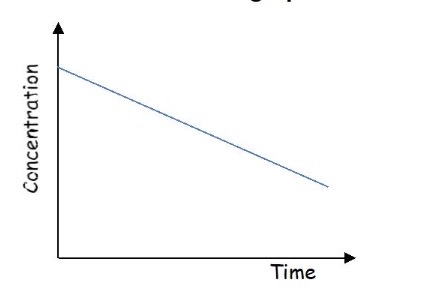
Determine the order from this graph + explain why
It’s a straight line
As concentration changes the gradient, the rate doesn’t change → the order with respect to this reactant is zero
For zero order reactions, what is the rate equation?
Rate = k
*therefore, the rate constant, k, can be determined by calculating the gradient
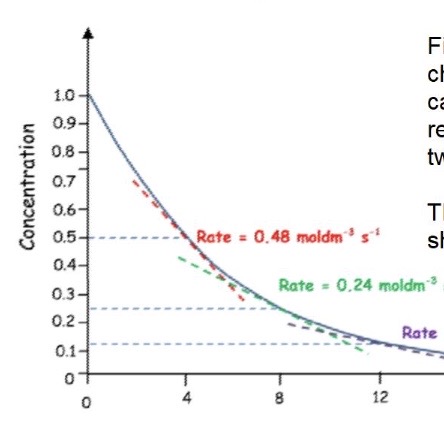
What is the order of this reaction + how can you tell?
First order reactions will have a curve as the rate change will equal the change in concentration → use the gradients to work out the order of reaction - should calculate at 2 points
Change in concentration between the 2 points should be equal to the change in rate for both points
Rate ∝ concentration
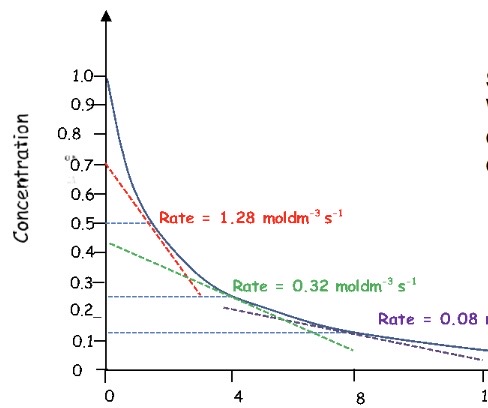
What is the order of this reaction + how can you tell?
Second order reactions also have a curve
When the gradient of 2 points on the curve are compared, the change in rate should equal the change in concentration squared
Rate ∝ concentration²