chemical toxicology flashcards
1/104
Earn XP
Description and Tags
lol i love feeling weird
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
105 Terms
non polar groups
lipophilic (fat loving)
hydrophobic
soluble in np solvents including fats
assessing lipophilicity
kow = conc of solute in octanol/water
kow > 5000 or log10kow > 3.7 = molecule is likely to bioconcentrate (membrane permeable)
negative kow = higher affinity for water
benzene logkow
2.13
napthalene logkow
3.37
anthracene logkow
4.5
phenanthrene logkow
4.5
pyrene logkow
4.88
benzo(a)pyrene logkow
6.1
benzo(e)pyrene logkow
6.2
chlorinated/brominated molecules
very lipophilic/persistent
increase london disp forces
phenol (1.49)→ pcp (logkow 5.12)
biphenyl (4.01)→ pcb (logkow 5.82)
hydrophilic substances
water loving
polar groups such as -oh, -nh2, co2h
soluble in water
not readily taken up in gut, excreted in urine
london dispersion
instant dp induced attactions that occur btwn all molecules even non polar
dipole dipole interactions
instant dp induced attractions that occur btwn all molecules even non polar, stronger than disp forces
pi stacking interactions
pi pi stacking
attractive noncovalent interactions btwn aromatic rings as they contain pi bongs
sandwich, tshaped, parallel displaced
h bonding
dp dp attraction when h is covalently bonded to highly electronegative atom
f > o > n
boiling point trends
group iv = no hbond, increase due to molecules getting larger w more electrons
h20/hf = more heat energy needed to break = hbond
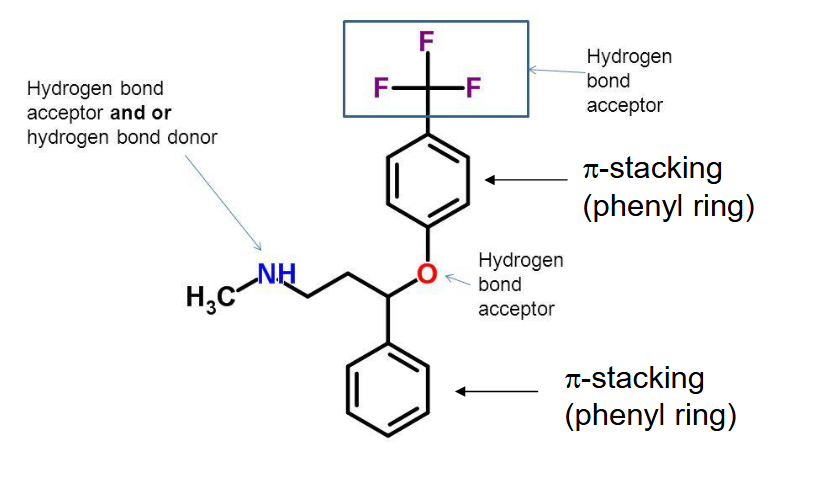
prozac
hbond acceptor/donor
pi stacking
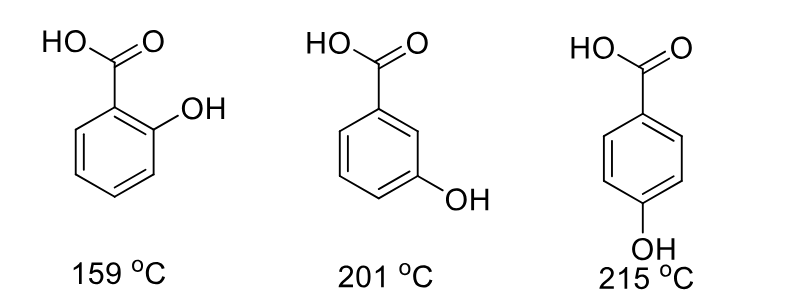
hydroxybenzoic acid derivative example
weaker = pi stacking
stronger = intermolecular hbond
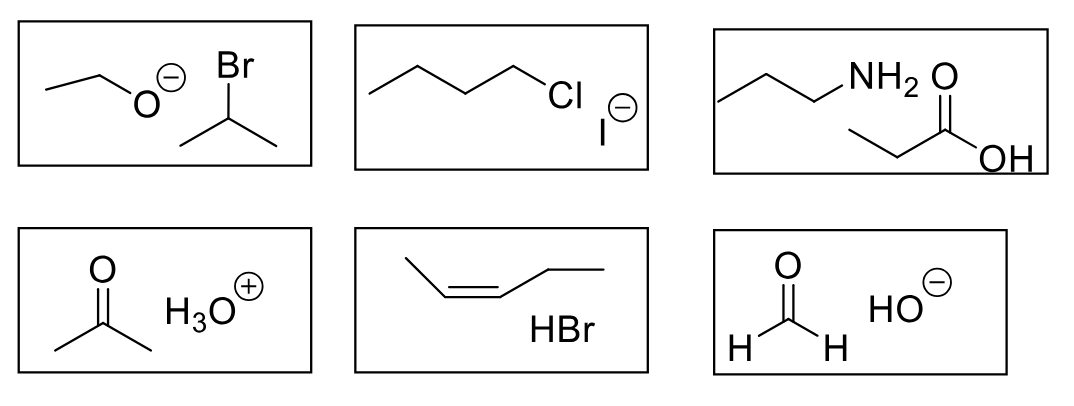
alcohol (p) + alkyl halide (np)
ether (lp) + hbr
alcohol (p) + carboxylic acid (p)
ester (lp) + h2o
alcohol (p) + aldehyde (lp)
carboxylic acid (p)
functional group conversions
morphine → heroin (oh to ester)
bronsted-lowry acid
proton donor (h+)
bronsted-lowry base
proton acceptor (h+)
heterolytic bond cleavage
RH → R- + (H+) proton
RH → R+ + (H-) hydride
homolytic bond cleavage
RH → Radical + (H) h atom
ph scale
basic/neutral for solution
acid dissociation - ka
ka = h3o+a-/ha
stronger aid = more h30 = larger ka
strong acid pka value
small positive or negative
weak acid pka value
large pka value
weak acid examples
carboxylic acid: rco2h pka = logkA
water (10)
phenol (15.7)
ethanol (16)
organic base
amines
ammonium ion → ammonia (conj acid → conj base)
surfactant
surface active agent
amphiphilic structure
np/p
anionic surfactant
negative charge
cationic surfactant
positive charge
amphoteric surfactant
positive/negative charge
nonionic surfactant
no charge
surfactant behavior
hydrophobic group distorts structure of water
some molecules expelled to surface of system w hydrophobic groups
micelle formation: polar solvent
monomers → spherical micelles → cylindrical micelles → bilayer lamella
micelle formation: non polar solvent
monomer → reverse micelles → inverted hexagonal phase
soaps/detergents
saponification
triglyceride → sodium salt + glycerol, glycerin
bio surfactants: bile salts
aids with digestion to emulsify lipids
bile salt accumulation = acutely toxic
acid/base properties of xenobiotics
uncharged = more lipophilic, easily taken up from gi tract
carboxylic acid/phenols = acid conj uncharged, basic conj ionic
nitrogen base = acidic cong ionic, basic conj uncharged
pka = acidity of toxicant
oral cavity pka
6.8 -7.5
stomach cavity
1.5-2.0
duodenum pka
5.8-8.0
small intestine pka
7.2-7.5
colon pka
7.9-8.5
covalent binding interactions
activated drug + target dna/protein/etc → adduct
adduct (addition product formation) can occur when drug is highly reactive (electrophile) and can covalently react w nucleophilic sites on target
nucleophiles
electron rich molecules that have one unshared pair of electrons, negatively charged
electrophiles
accepts electrons as they are lacking an e
4 key factors that contribute to nucleophilicity
charge: conj base better nucleophile (nucleophilicity inc w inc electron density)
electronegativity: nucleophilicity increases with decreasing electronegativity (les er, less held electrons are)
solvent
polar protic (water): can h bond creating a shell of solvent molecules, nucleophile: less reactive as lp interacts with h atoms of solvent
aprotic: lack hbond, nucleophilicty increases going up periodic table (f(strongest)>cl>br>i)
steric hindrance: bulkiness
reactive intermediates
electrophiles
carbon/nitrogen atoms attached to leaving group X (weak bases)
results in bond polarization, nucleophile drawn to partially positive c atom\
sn2 = one bond broken/one formed in one step (nucleophilic substitution)
carbocations (3>2>1>methyl)
sn1 = nucleophile replacing leaving group, unimolecular and rate of rxn depends on concentration of one reactant (2 step)
carbonyl compounds
alpha,beta-unsaturated carbonyl compounds
quinones: unique class of carbonyl compounds
epoxides
all reactions involve nucleophilic attack at carbon and lead to opening of ring
driving force = steric strain
question
(-) gain e (anion)
(+) lose e (cation)
radicals
have one unpaired e + v reactive

radical reactions
addition (r. +ch2=ch2 → rch2ch2.)
hydrogen abstraction (r. +LH → RH + L.)
electron abstraction (r. +ArNH→r- +ArNH+.)
termination (r.+y.→r-y)
disproportionation (ch3ch2r+ch3ch2r =ch3ch3 + ch2=ch2)
oxidative stress
excess of prooxidant/antioxidant reactive species
detrimental consequences (cancer/aging)
results from exposure to ros
antioxidants combat oxidative stress
oxidative defense
small molecules
water soluble: glutathione, uric acid, ascorbate
lipid soluble: alpha-tocopherol, beta-carotene, coenzyme q
proteins
intracellular: sod 1/2, glutathione peroxidase, catalase
cell membrane: sod 2, exGPX, plasma proteins
extracellular: phospholipid hydroperoxide GPx
metabolism
allows for enzymatic conversion of chemicals to polar compounds which are easily excreted
most metabolites = water soluble due to addition of hydrophilic func groups
more ionized than parent @ phys ph
rendered less biologically active/inactivated
alcohol toxicology
ethanol (alcohol dehydrogenase) → acetaldehyde
acetaldehyde (aldehyde dehydrogenase) → acetic acid
95%, 5% in urine/feces/breath/sweat
therapeutic agents & metabolism
need to be metabolized to be active
proximate carcinogens (parent not carcinogenic)
render more polar → concert to highly reactive electrophiles
liver = xenobiotic metabolism primary site, largest concentration of metabolic enzymes
first pass effect: pass through liver before target site (activated/inactivated)
phase 1 metabolism summary
cytochrome p450
types of reactions: hydrolysis, oxidation,reduction
small inc in polarity
exposes functional group
can result in metabolic activation
phase 2 metabolism summary
conjugation pathways
type of reaction: conjugation
large inc in polarity
polar compound added to functional group
facilitates excretion
cytochrome p450
enzyme family responsible for biotransformation rxn used to metabolize endo/exogenous compounds
microsomal resulting from fragmentation of endoplasmic reticiulm
mixed functional oxidases/monooxygenase because they catalyze insertion of one molecule of oxygen into substrate, one o atom is inserted into chemical and other is reduced to form h2o
spectral properties
when reduced to ferrous can bind ligands like o2/co
cyp450 absorbs light maximally @ 450 nm due to 5th ligand to the heme
cytochrome p450 oxygen rebound mechanism
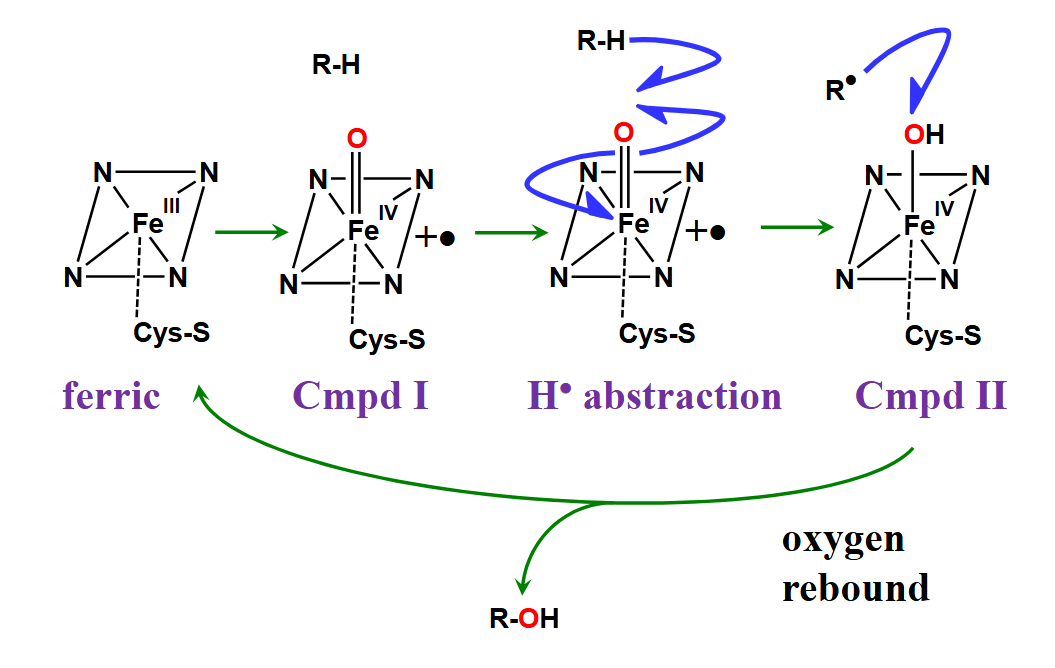
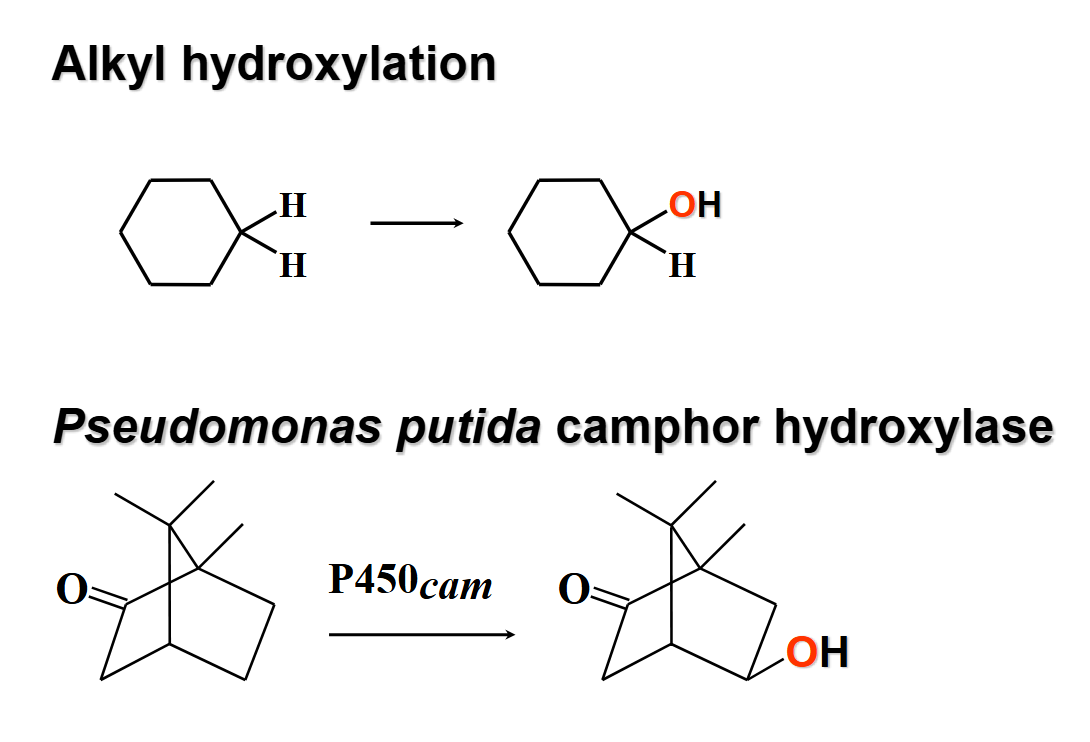
cytochrome p450 - ppc hydroxylase
-oh replace -h
ch bond dissociation energy
1: 410 kj/mol
2: 395
3: 380
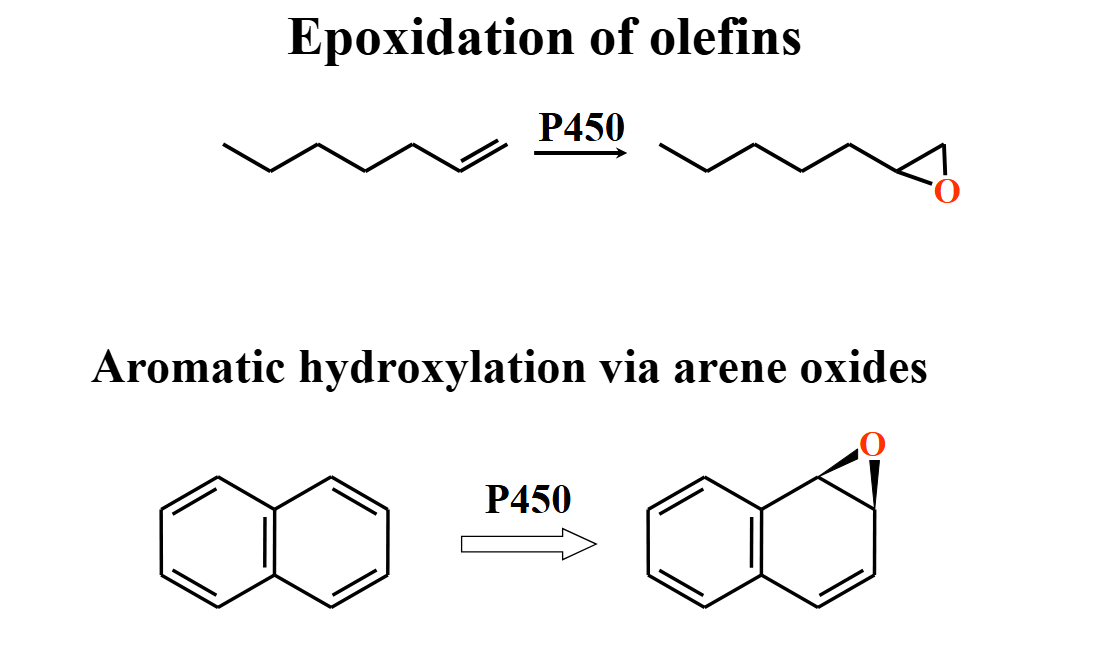
epoxidation of olefins
undergo ring opening through reactions w nucleophiles
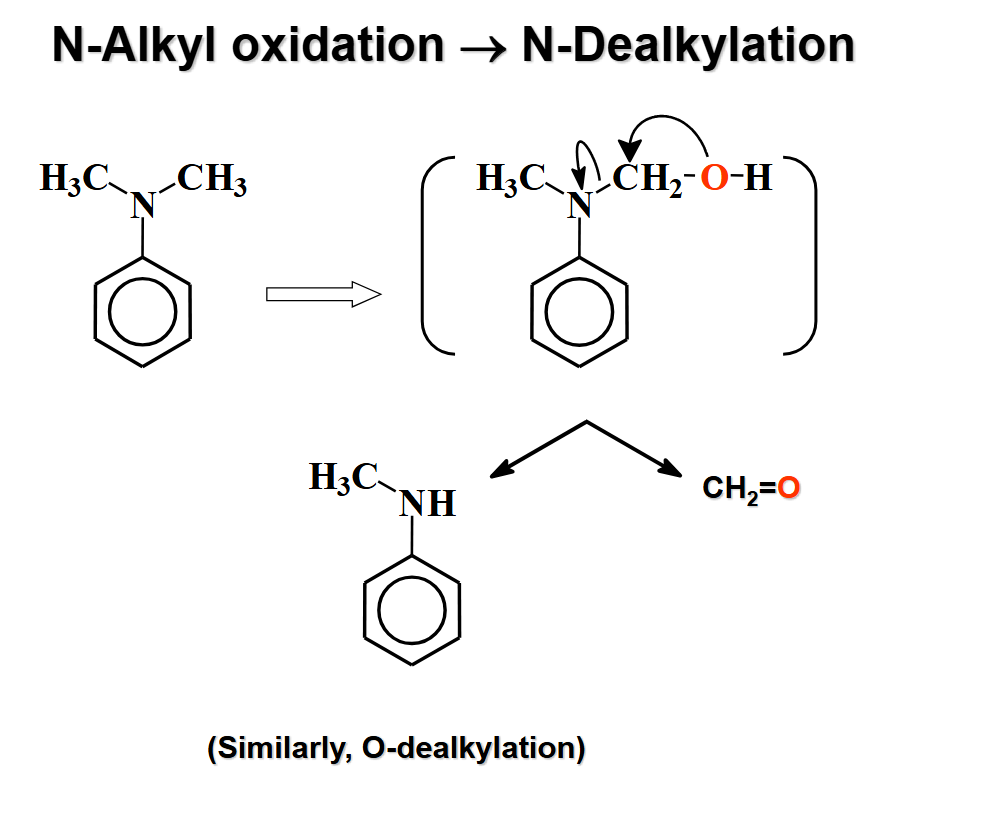
N-alkyl oxidation
ch2=o + h-r
oxycodone p450 metabolism
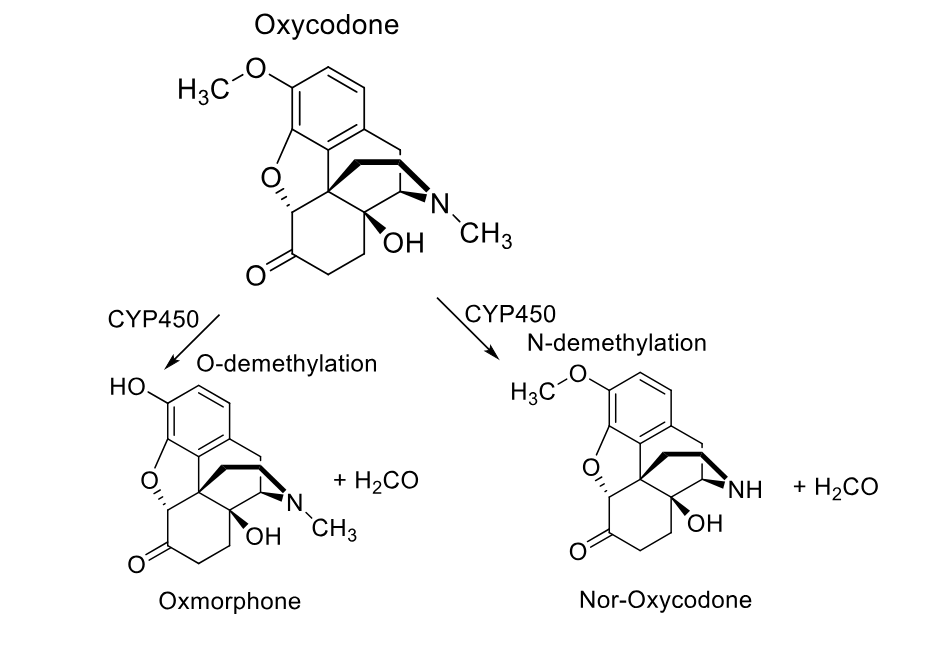
phase 2 metabolism
couples metabolite w endogenous substrate resulting in large inc in metabolite polarity
increased metabolite stability
eliminate reactive intermediates
phase 2 reactions conjugation
glucuronic acid - glucuronides
acetyl coenzyme a - acetylated
paps - sulfonated
glutathione - mercapturic acid
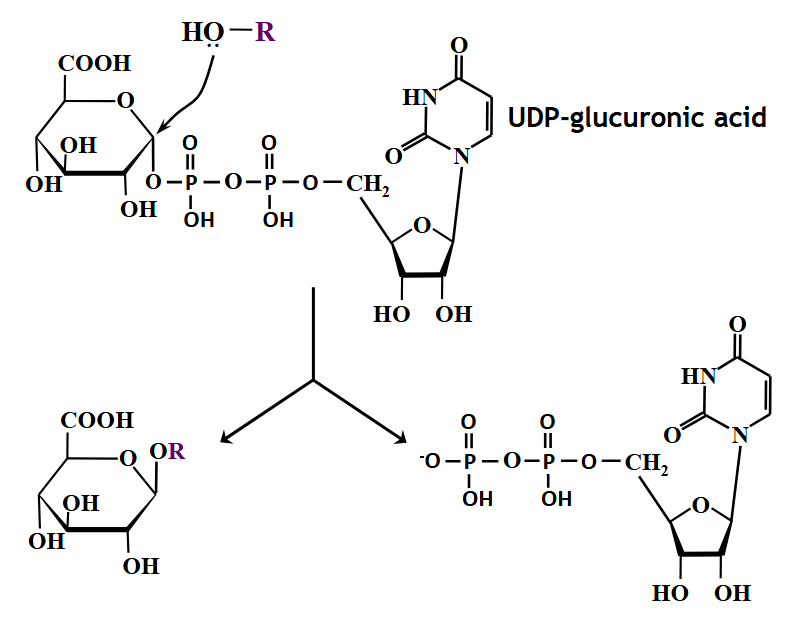
glucuronidation
nucleophile (roh)
UDPGA cofactor
UDP-glucuronosyl transferase (ugt)
N-acetylation
nucleophile (rnh2)
acetyl-coa cofactor
N-acetyl-transferase (nat)
sulfation
nucleophile (roh)
paps cofactor
sulfotransferase (sult)
glutathione conjugation
electrophile (epoxide)
gsh cofactor
glutathione s-transferase (gst)
udp-glucuronosyltransferases
aglycone + UDPGA → glucuronide + UDP
glucuronic acid = main sugar used to prevent accumulation of waste products of metabolism and fat soluble chemicals from the environment
UDPGT
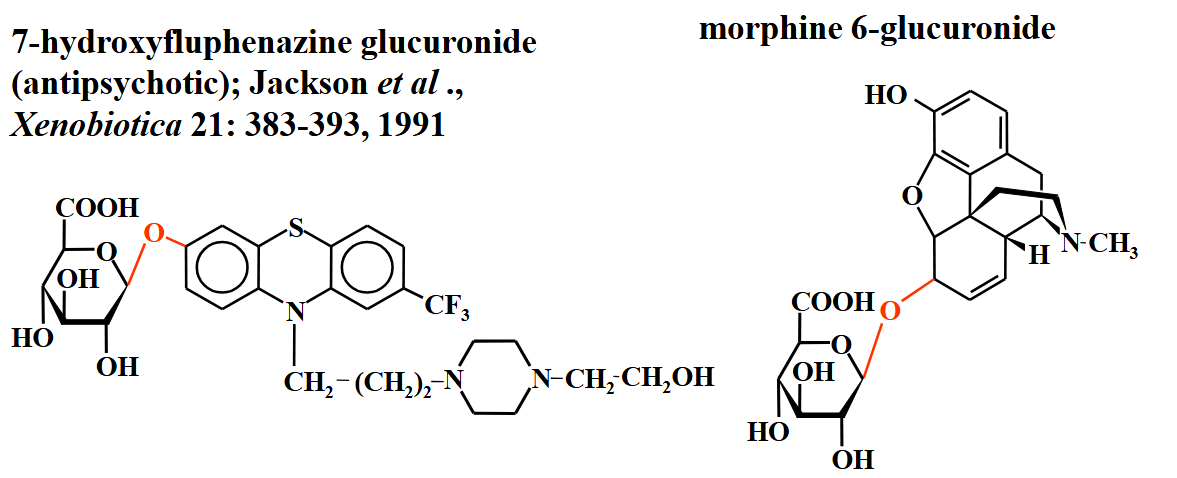
glucuronide structures
o-glucuronides
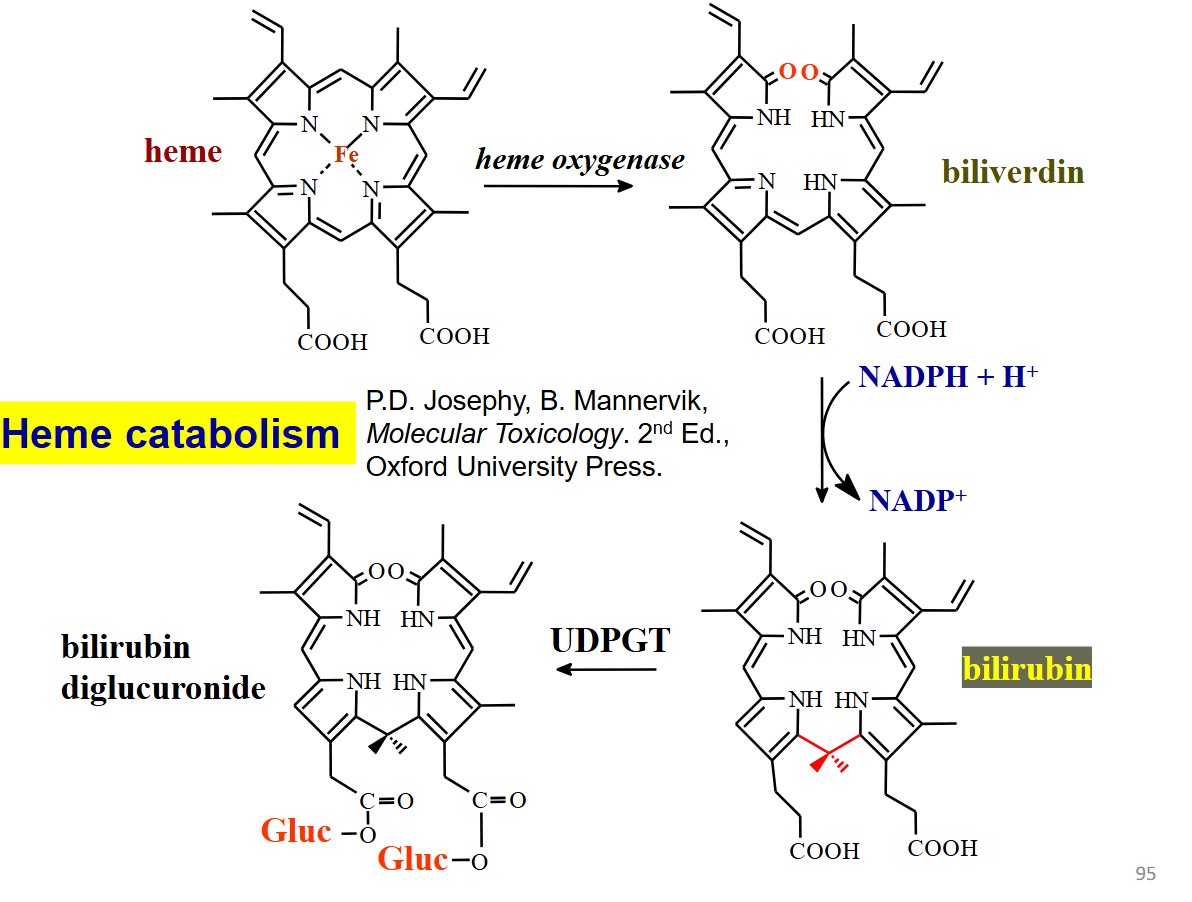
heme catabolism
heme → biliverdin (heme oxygease)
biliverdin → bilirubin (nadph + h+ → nadp+)
bilirubin → bilirubin diglucuronide (UDPGT)
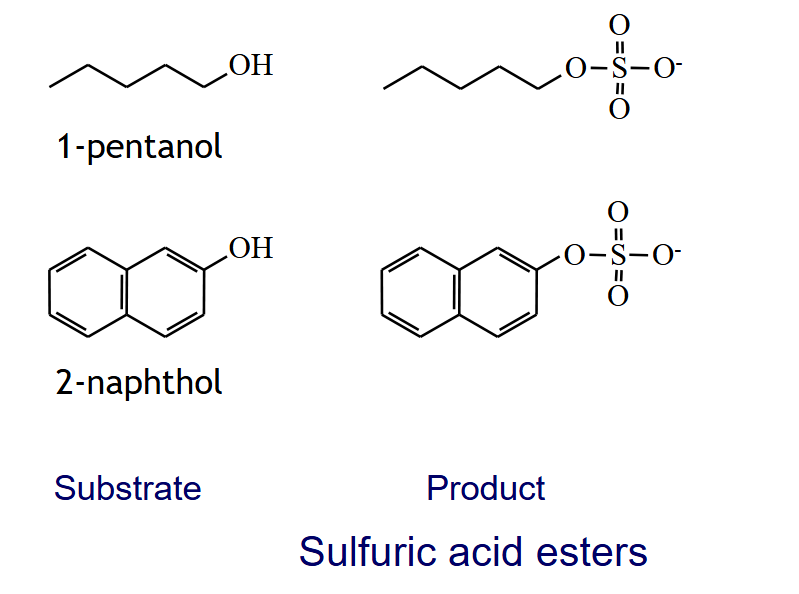
sulfotransferase
R-OH + PAPS → R-OSO3- + PAP
SULFURIC ACID ESTERS
acetyl coa: NAT
substrates
aromatic amines ar-nh2
aromatic hydrazines ar-nh-nh2
drugs: isoniazid, sulfamethazine, procainamide
Synthetic intermediates: benzidine
Food pyrolysis product heterocyclic amines

gsh - glutathione gamma-glutamylcysteinylglycine
gamma peptide linkage prevents hydrolysis by peptidases
ionized thiolate (gs-) is a good nucleophile
sulfur to donate e makes gsh good reductant
GSH → GS- + H+ (pKA: 9.2)
gsh as a reducing agent and free radical scavenger: defense against oxidative stress
gsh reacts w many electrophiles to form covalent adducts
gsh as nucleophile
toxicant →(gsh-gsx adduct = stops toxicant) reactive metabolite (electrophile) → biological target (nucleophile) & macromolecule (dna/rna/protein) → covalent adduct → biological damage
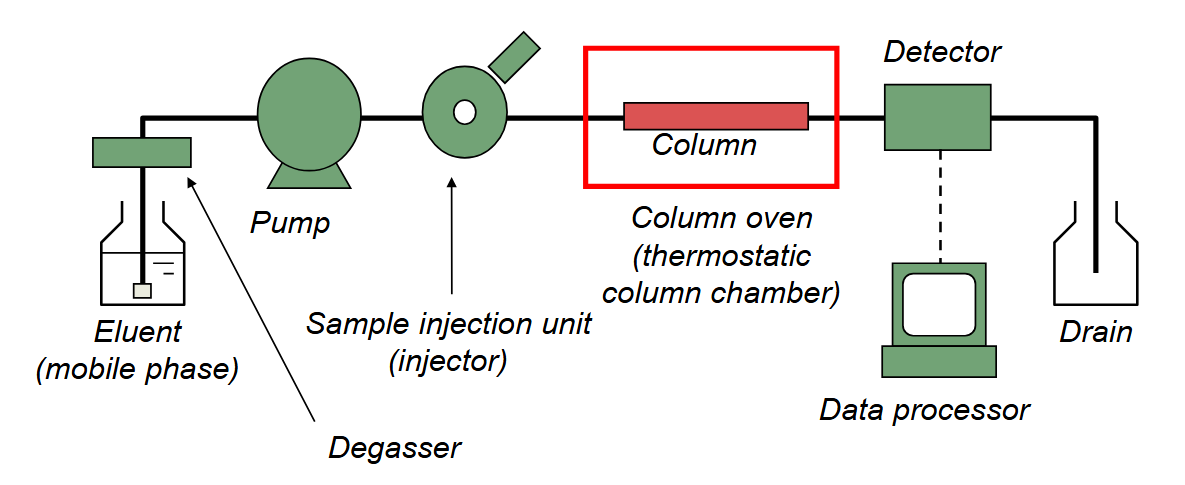
HPLC W MS LCMS
high pressure liquid chromatography w mass spectrometry detection lcms
understanding activity/safety of key metabolites critical to overall evaluation of drug product
mobile/stationary phase
mobile/stationary phase make contacty in interface set up
affinity varies w solute
separation occurs due to differences in speed of motion
column and planar chromatography
LC to HPLC
higher degree of separation
reduction of analysis time
eluent by pump
gradient system
isocratic system
constant eluent composition
long analysis time + poor separation
gradient system
varying eluent system (HPGE/LPGE)
eluent comp changed gradually
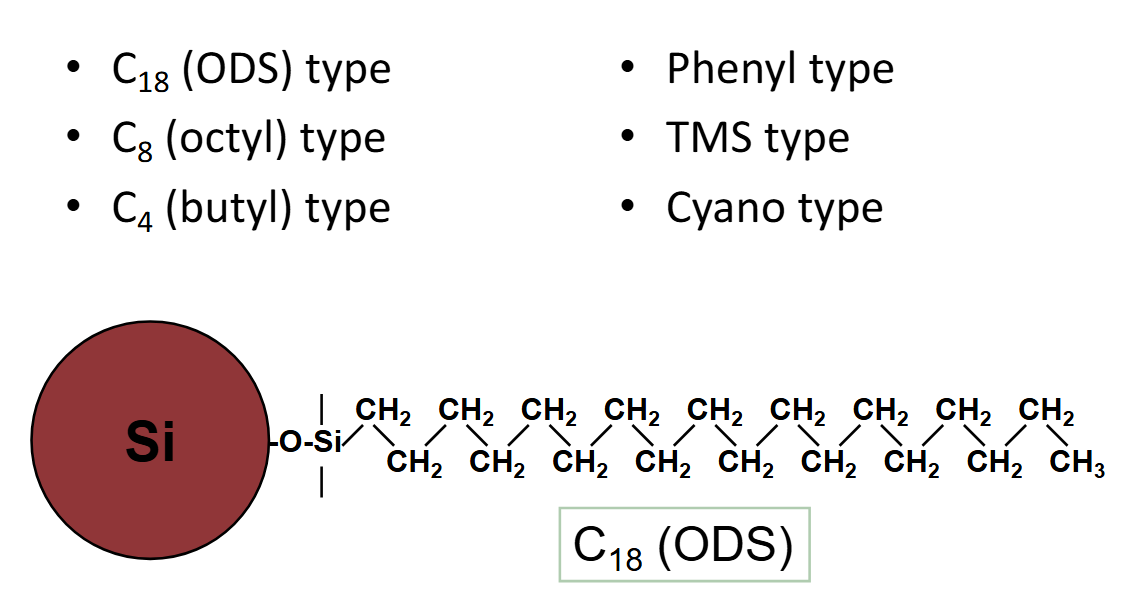
reversed phase chromatography
stationary phase: low polarity
ods
mobile phase
high polarity
water/methanol/acetonitrile/salt sometimes added
separation column for reverse phase chromatography
eluent used in reversed phase mode
water + water soluble organic buffer
ratio has greatest influence on separation, ph is important separation parameter
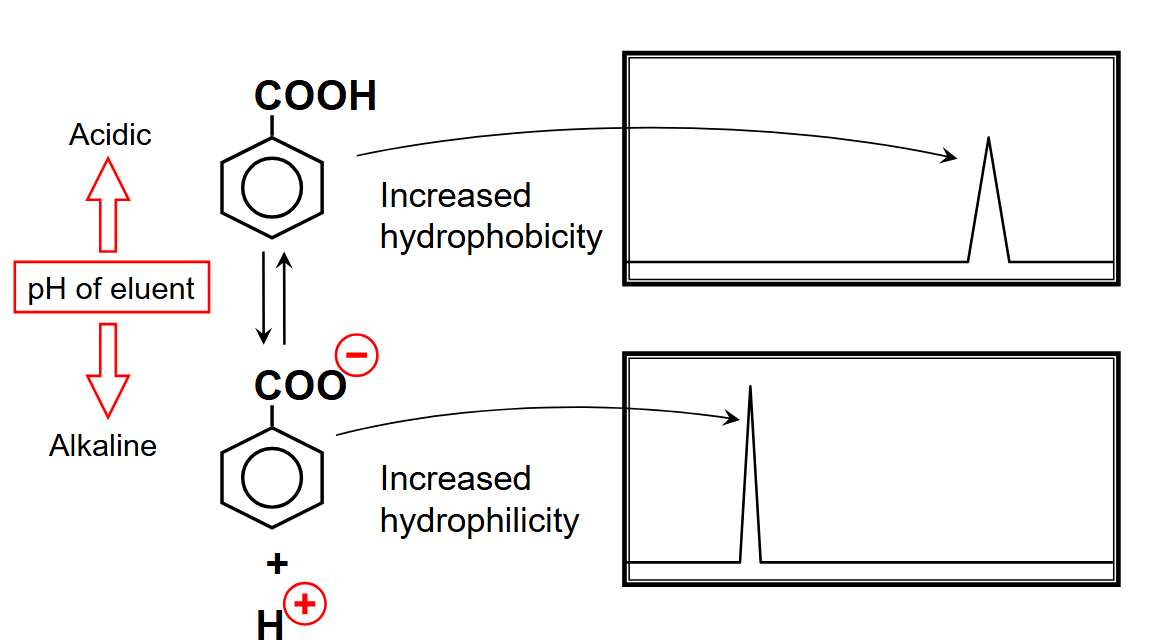
pH of eluent/retention of ionic solutes
normal phase: most non polar first (stationary: polar, mobile: np)
reverse phase: most polar first (stationary: np, mobile: polar)
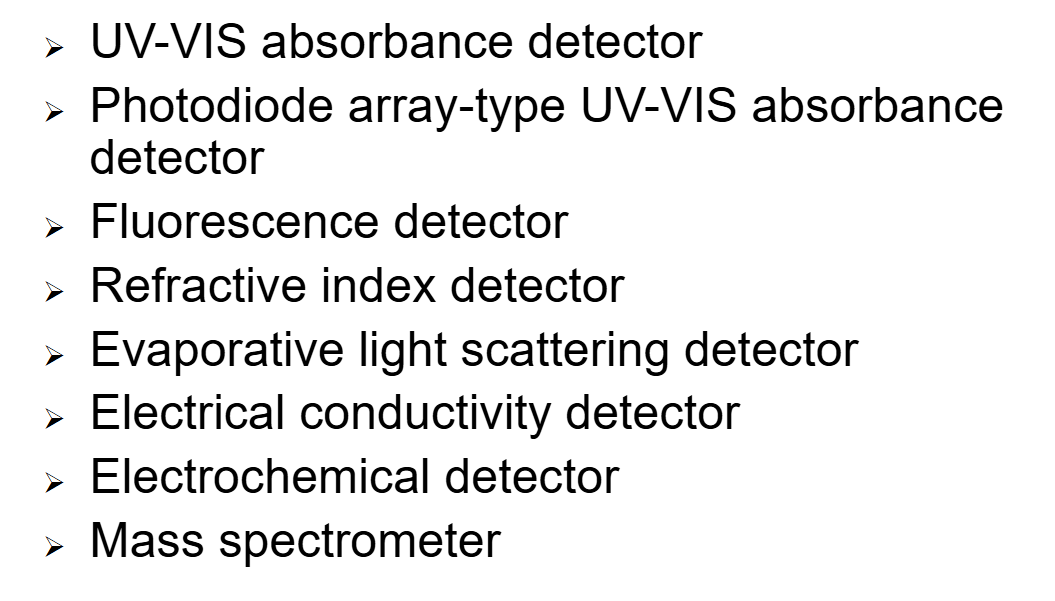
HPLC DETECTORS
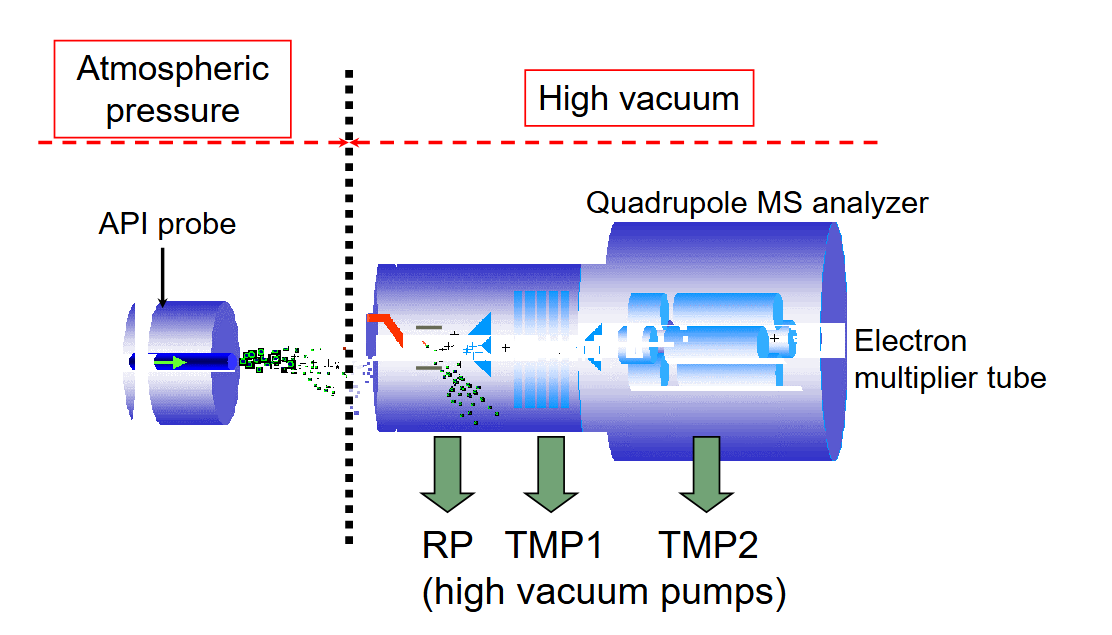
mass spec lcms
base peak = most intense peal
ms parent ion = mwt of compound
lcms advantages
can be id’d w ms spectra & quantitve analysis w excellent selectivity
detection limit
minimum quantity of target substance that can be detected
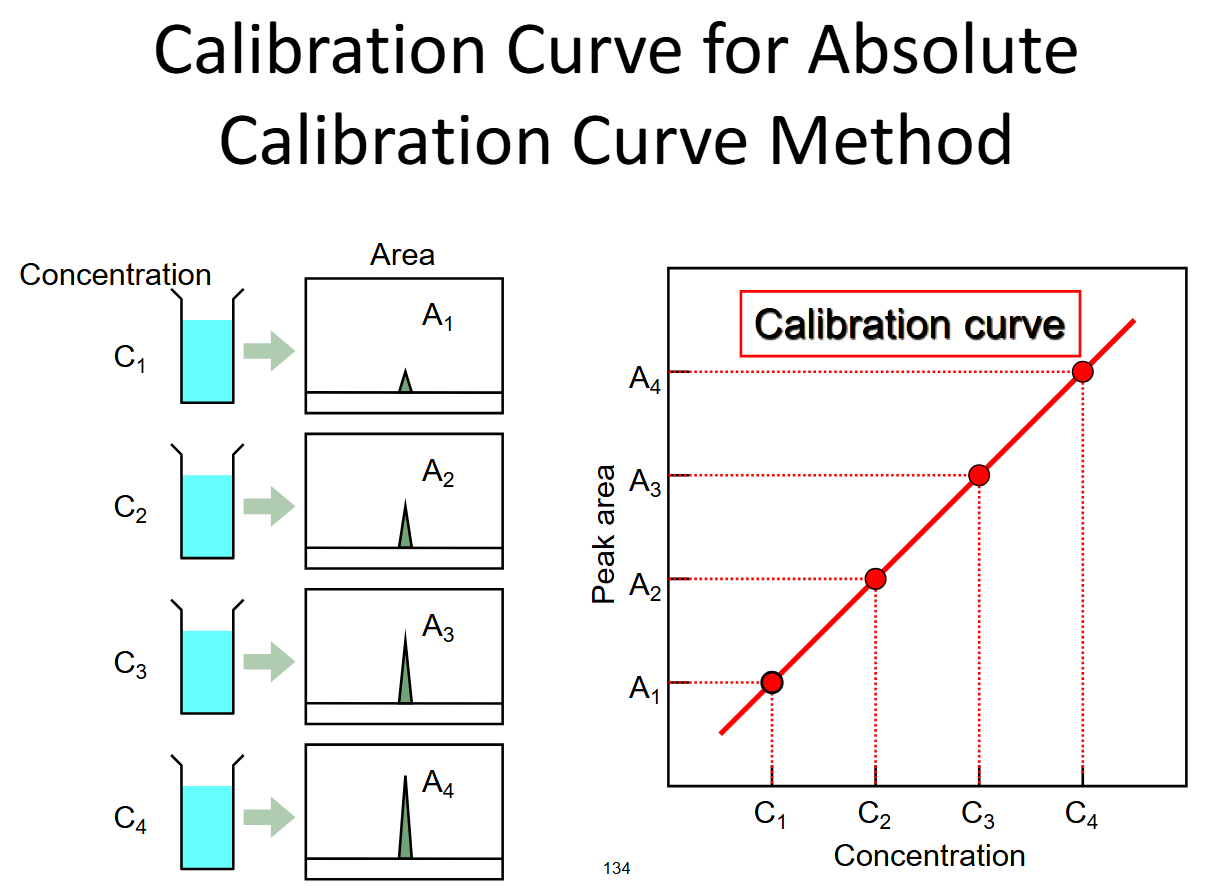
evaluation method
calculated from sd of measurements and slope of calibration curve
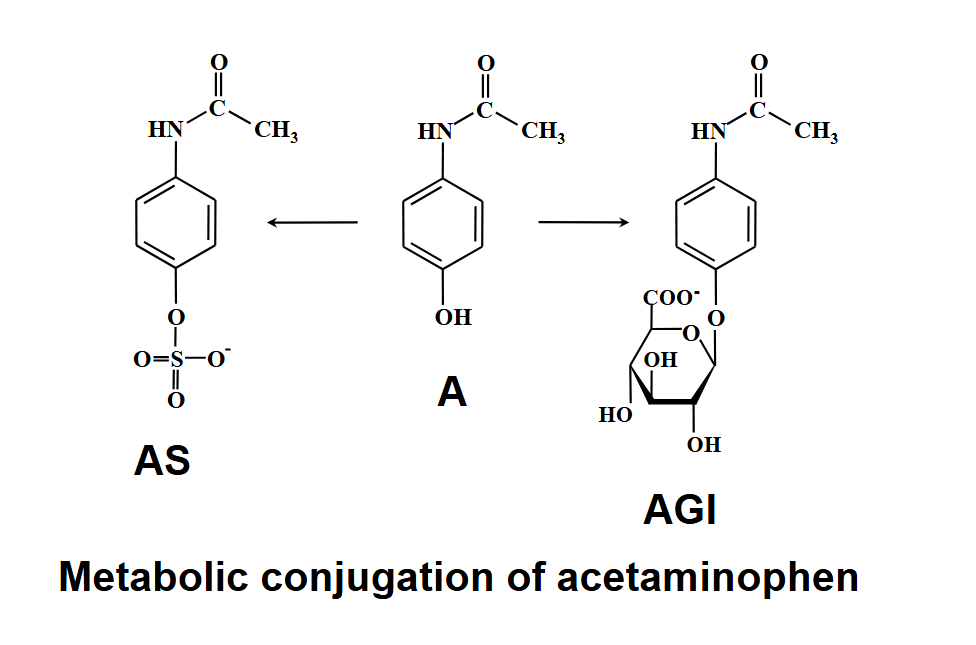
acetaminophen
hepatotoxicty = most common cause of acute liver failure