graphite
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
key properties of graphite
soft & slippery
has a very high melting and boiling point
good conductor of electricity and heat
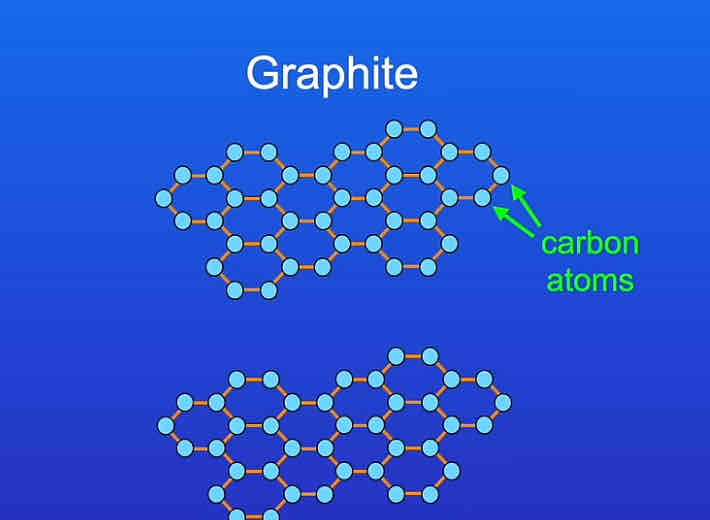
what is graphite formed from?
the element carbon
in graphite what does each carbon atom form?
forms covalent bonds to three other carbon atoms.
what do the carbon atoms form?
hexagonal rings (rings of six carbon atoms).
what are the hexagonal rings of carbon atoms arranged into?
layers
there are no what between the layers?
no covalent bonds
due to no covalent bonds between the layers what does this mean and what does this make graphite?
this means the layers can slide over each other, this makes graphite soft and slippery.
what is graphite often used as?
a lubricant in machines reducing friction between the moving parts.
what does graphite contain?
a large number of strong covalent bonds.
if we want to melt graphite what do we need to do and what does this explain about graphite?
break those strong covalent bonds, this takes a great deal of energy.
this explains why graphite has a high melting and boiling point.
graphite is formed from?
carbon atoms
how many electrons do carbon atoms have in their outer energy level?
four electrons
in graphite each carbon atoms forms covalent bonds to how many other carbon atoms?
three other carbon atoms
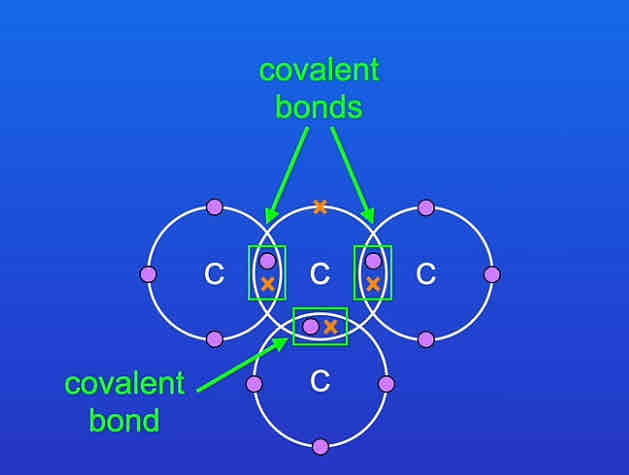
each carbon atom has how many electrons in its outer energy level and it’s not in what?
one electron and it’s not in a covalent bond.
where are these electrons released from and what are they called?
the carbon atoms, they are called delocalised electrons.
these delocalised electrons can move meaning what and what does this make graphite similar to?
this means they can conduct both electricity and thermal energy (heat), this makes graphite similar to metals.
just like graphite what do metals also have?
delocalised electrons that can move.
like graphite what are metals good conductors of?
electricity and heat.
why is graphite not a metal?
as it’s formed from carbon.