Comprehensive Biology: Chemistry of Life, Molecules, and Cell Functions
1/307
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
308 Terms
Biochemistry
The study of the molecules that compose living organisms.
Chemical Element
The simplest form of matter to have unique chemical properties.
Atomic Number
Number of protons in the nucleus of an atom.
Periodic Table
Arranges elements by atomic number and represents them as 1-2 letter symbols.
Naturally Occurring Elements
91 elements that are found in nature.
Most Abundant Elements in Humans
6 elements that make up 98.5% of body weight: oxygen, carbon, hydrogen, nitrogen, calcium, and phosphorus.
Trace Elements
Elements present in minute amounts that play vital roles in the body.
Minerals
Inorganic elements extracted from soil by plants and passed up the food chain to humans.
Body Weight Contribution of Minerals
4% of body weight, mostly calcium and phosphorus.
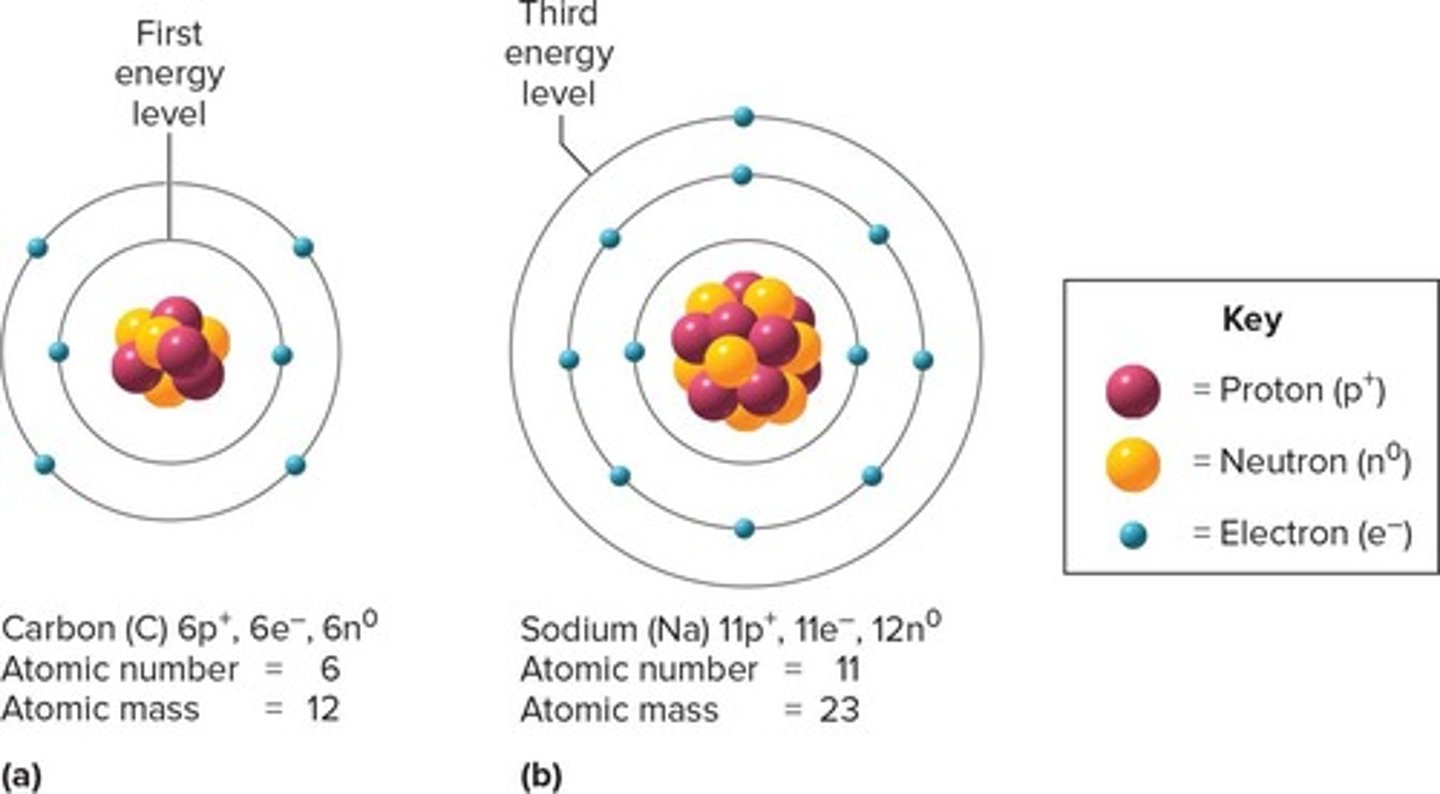
Atom
The smallest unit of matter.
Nucleus
Center of an atom, composed of protons and neutrons.
Protons
Particles with a single (+) charge and a mass of 1 atomic mass unit (amu).
Neutrons
Particles with no charge and a mass of 1 atomic mass unit (amu).
Electrons
Particles with a single (−) charge and very low mass, found in concentric clouds surrounding the nucleus.
Atomic Mass
Approximate total number of protons and neutrons in an atom.
Valence Electrons
Electrons in the outermost shell that determine the chemical bonding properties of an atom.
Isotopes
Varieties of an element that differ only in the number of neutrons and therefore in atomic mass.
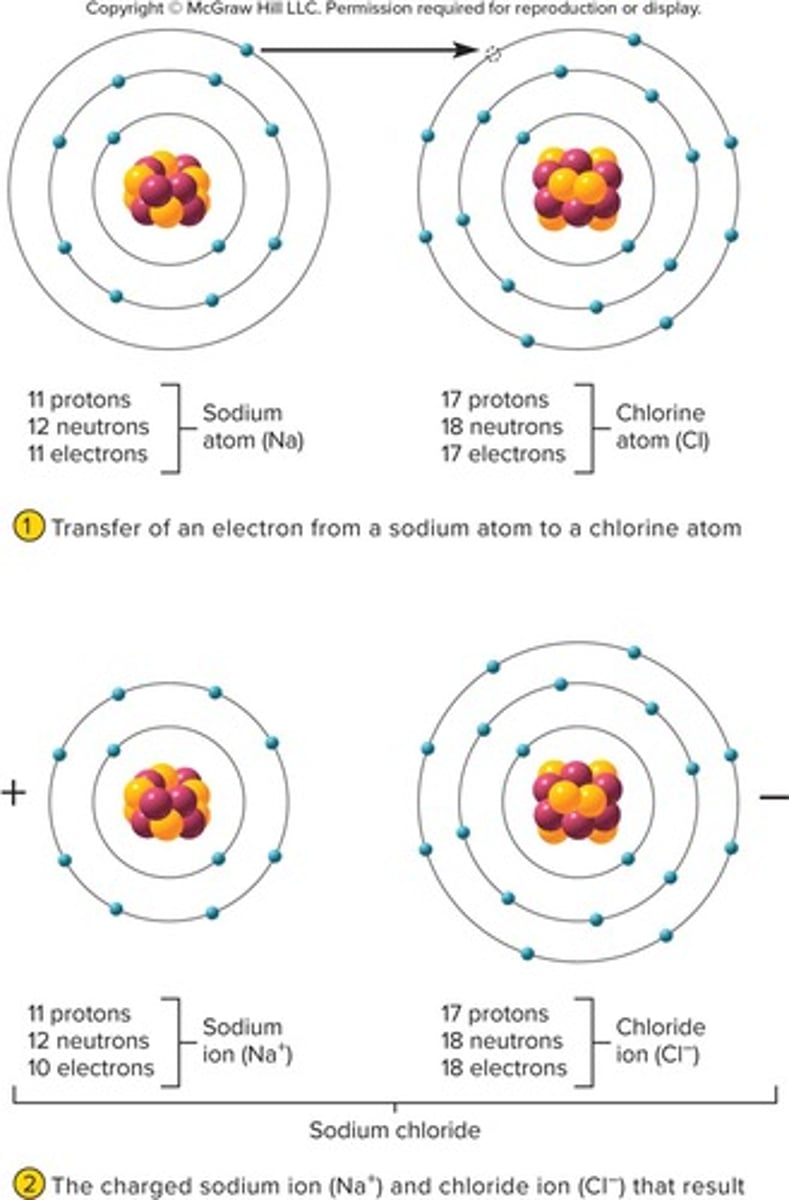
Ion
A charged particle (atom or molecule) with an unequal number of protons and electrons.
Ionization
Transfer of electrons from one atom to another.
Anion
A particle that has a net negative charge due to the gain of electrons.
Cation
A particle that has a net positive charge due to the loss of electrons.
Ions with Opposite Charges
Attracted to each other.
Salts
Electrically neutral compounds of cations and anions; readily dissociate in water into ions and act as electrolytes.
Electrolytes
Substances that ionize in water and form solutions capable of conducting electric current.
Functions of electrolytes
Chemical reactivity, osmotic effects, electrical excitability of nerve and muscle.
Electrolyte balance
One of the most important considerations in patient care; imbalances can lead to coma or cardiac arrest.
Free radicals
Unstable, highly reactive particles with an unusual number of electrons.
Production of free radicals
Produced by normal metabolic reactions, radiation, certain chemicals.
Effects of free radicals
Trigger reactions that destroy molecules, and can cause cancer, death of heart tissue, and aging.
Example of a free radical
Superoxide anion.
Antioxidants
Chemicals that neutralize free radicals.
Example of an antioxidant
Superoxide dismutase (SOD) is an antioxidant enzyme that converts superoxide anion into oxygen and hydrogen peroxide.
Dietary antioxidants
Selenium, vitamin E, vitamin C, and carotenoids are antioxidants obtained through the diet.
Molecule
Particle composed of two or more atoms united by a chemical bond.
Compound
Molecule composed of two or more different elements.
Molecular formula
Represents a compound by identifying constituent elements and how many atoms of each are present.
Ionic bonds
Attraction of a cation to an anion.
Example of ionic bond
Sodium and chloride ions bond to form sodium chloride.
Covalent bonds
Atoms share one or more pairs of electrons.
Single covalent bond
Nuclei share 1 pair of electrons.
Double covalent bond
Nuclei share 2 pairs of electrons.
Nonpolar covalent bond
Electrons are shared equally; example: carbon atoms bonding together.
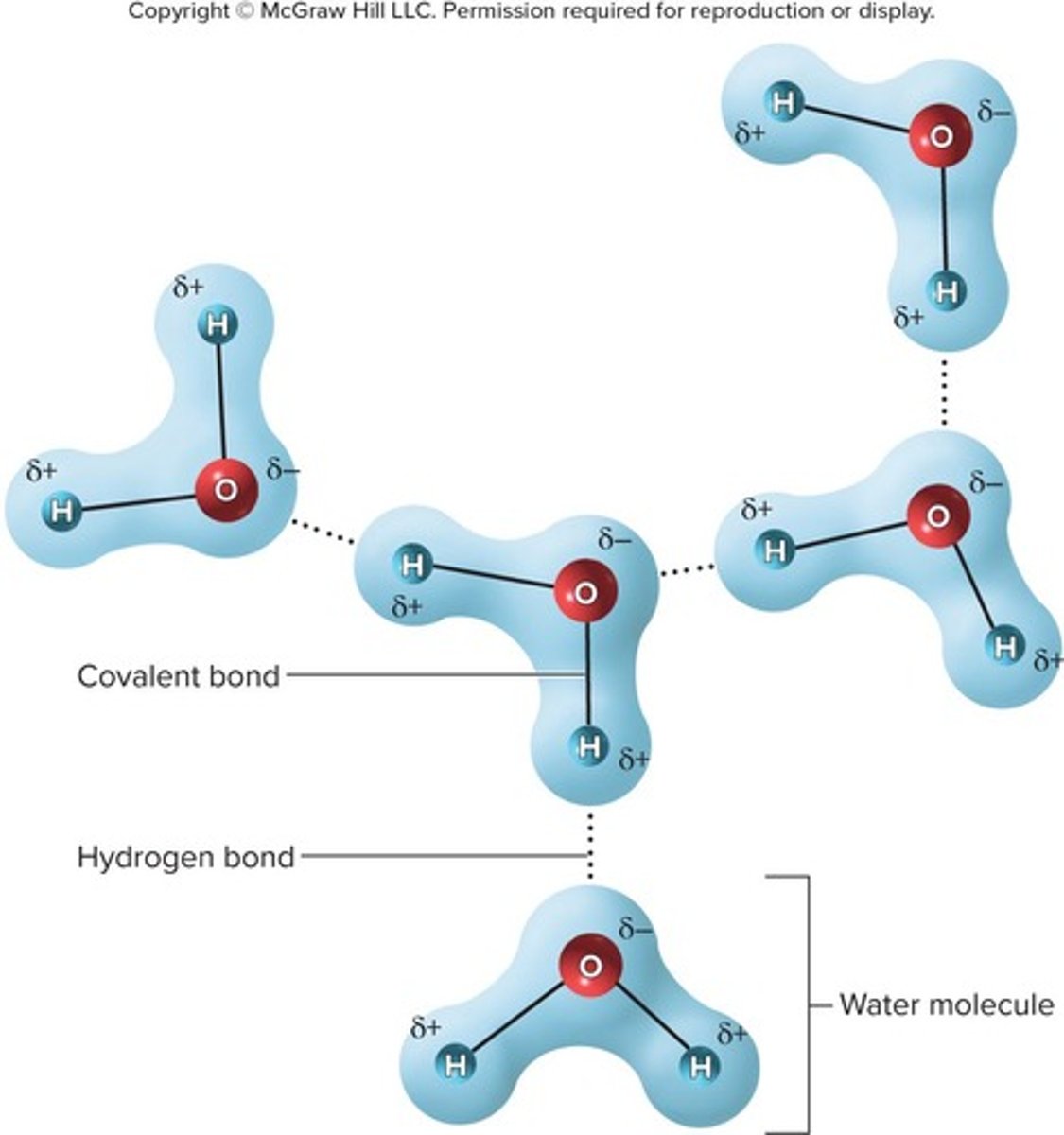
Polar covalent bond
Electrons shared unequally; example: hydrogen bonding with oxygen, electrons spend more time by oxygen.
Hydrogen bond
A weak attraction between a slightly positive hydrogen atom in one molecule and a slightly negative oxygen or nitrogen atom in another atom.
Importance of hydrogen bonds
Relatively weak bonds, but very important to physiology; water molecules are attracted to each other by hydrogen bonds.
Body fluids
Complex mixtures of chemicals.
Mixtures
Consist of substances that are physically blended but not chemically combined; each substance retains its own chemical properties.
Water
Makes up 50 to 75% of body weight and is essential for life.
Solvency
Ability to dissolve other chemicals.
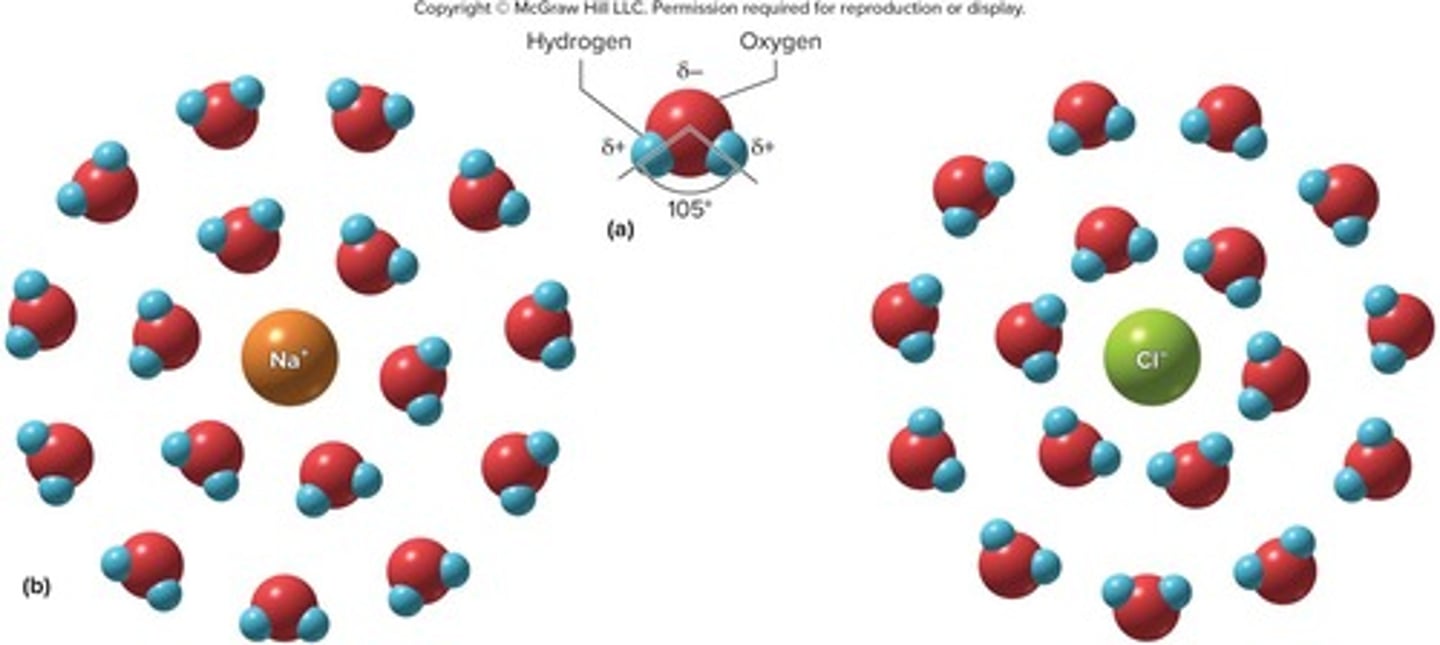
Universal solvent
Water is the universal solvent because it dissolves more substances than any other solvent.
Hydrophilic substances
Substances that dissolve in water; are polarized or charged.
Hydrophobic substances
Substances that do not dissolve in water; are nonpolar or neutral.
Hydration spheres
Water forms hydration spheres around each ion, allowing salts like NaCl to dissolve.
Adhesion
Tendency of one substance to cling to another.
Cohesion
Tendency of molecules of the same substance to cling to each other.
Chemical reactivity
Ability to participate in chemical reactions.
Thermal stability
Water stabilizes internal temperature; hydrogen bonds resist temperature increases.
Solution
Consists of particles called the solute mixed with a more abundant substance (usually water) called the solvent.
Solute
Can be gas, solid, or liquid that is mixed in a solution.
Colloids
Mixtures of protein and water that can change from liquid to gel state.
Suspension
Defined by particles too large to penetrate selectively permeable membranes and that separate on standing.
Emulsion
Suspension of one liquid in another, such as oil-and-vinegar salad dressing.
Acid
A proton donor; releases ions in water.
Base
A proton acceptor; accepts ions in water.
pH scale
Measures acidity based on the amount of hydrogen ions present.
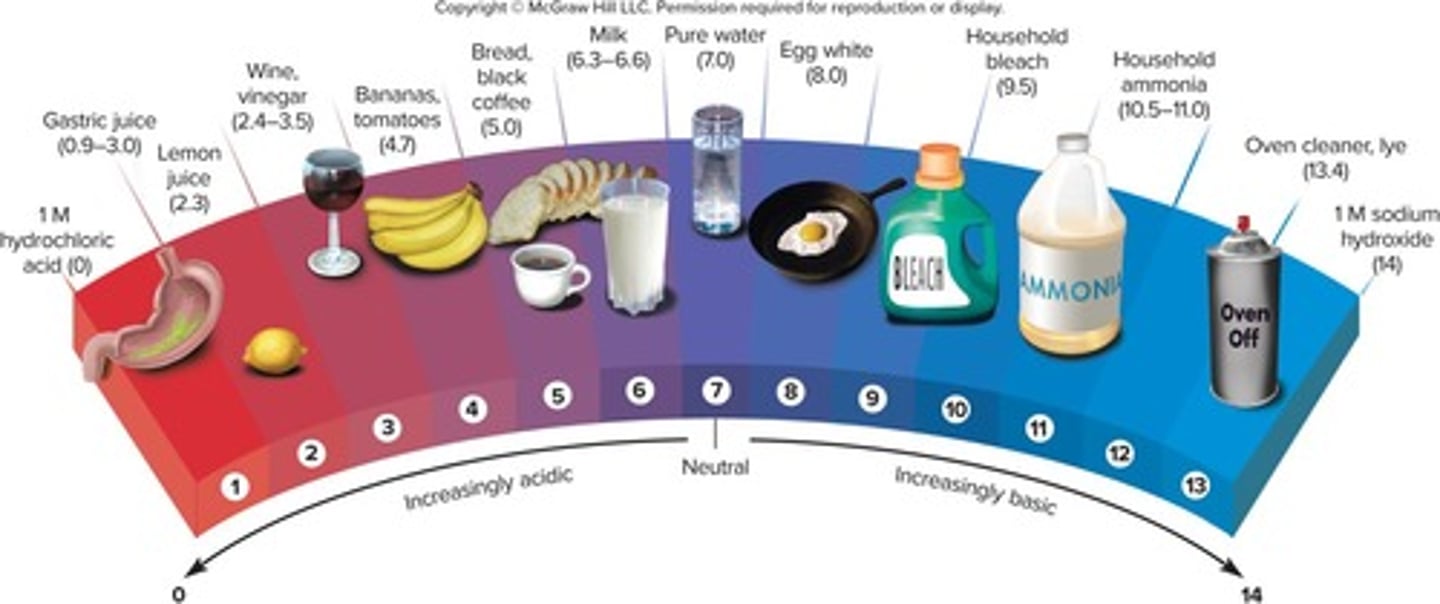
Neutral pH
A pH of 7.0.
Acidic pH
A pH of less than 7.
Basic pH
A pH of greater than 7.
Buffers
Chemical solutions that resist changes in pH.
Normal pH of blood
The normal pH of blood is 7.4, which is crucial for physiological functions.
Energy
The capacity to do work.
Work
To do work means to move something, such as a muscle or a molecule.
Potential energy
Energy stored in an object, but not currently doing work; for example, water behind a dam.
Chemical energy
Potential energy in molecular bonds.
Free energy
Potential energy available in a system to do work.
Kinetic energy
Energy of motion, energy doing work.
Heat
Kinetic energy of molecular motion.
Electromagnetic energy
Kinetic energy of photons.
Electrical energy
Energy that has both potential and kinetic forms.
Chemical reaction
A process in which a covalent or ionic bond is formed or broken.
Chemical equation
A symbolic representation of the course of a chemical reaction.
Reactants
Substances on the left side of a chemical equation.
Products
Substances on the right side of a chemical equation.
Decomposition reactions
Reactions where a large molecule breaks down into two or more smaller ones, symbolized as AB → A + B.
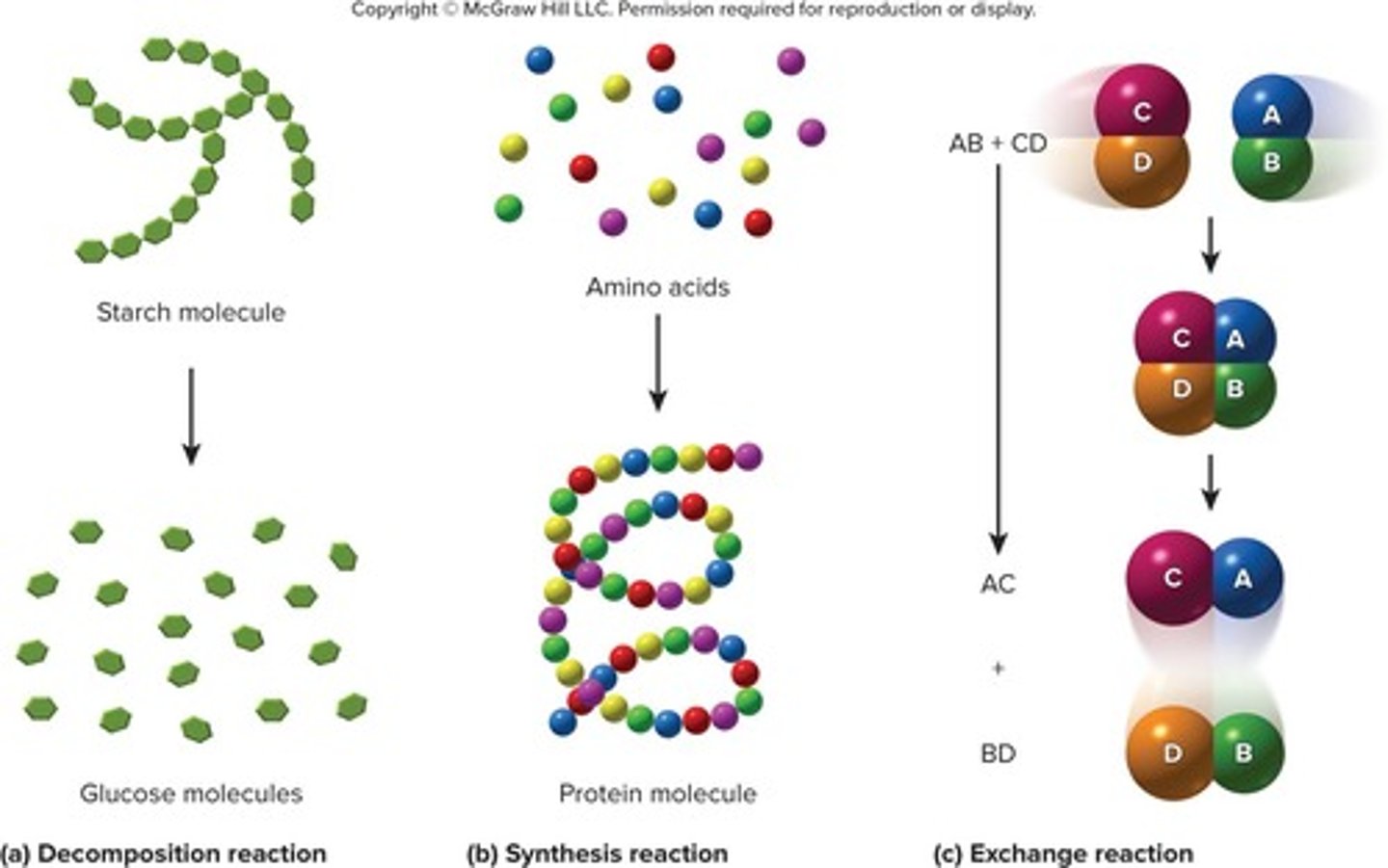
Synthesis reactions
Reactions where two or more small molecules combine to form a larger one, symbolized as A + B → AB.
Exchange reactions
Reactions where two molecules exchange atoms or groups of atoms, symbolized as AB + CD → AC + BD.
Reversible reactions
Reactions that can proceed in either direction under different circumstances, symbolized with a double-headed arrow.
Law of mass action
The principle stating that the direction of a reversible reaction is determined by the relative abundance of substances on either side of the equation.
Equilibrium
A state reached when the ratio of products to reactants is stable.
Reaction rates
The speed at which a chemical reaction occurs, which increases when the concentration of reactants increases, temperature rises, or a catalyst is present.
Catalysts
Substances that increase the rate of a reaction without being consumed in the process.
Metabolism
All chemical reactions of the body, divided into catabolism and anabolism.
Catabolism
Energy-releasing (exergonic) decomposition reactions that break covalent bonds and produce smaller molecules.
Anabolism
Energy-storing (endergonic) synthesis reactions that require energy input.
Organic chemistry
The study of compounds containing carbon.
Macromolecules
Large organic molecules with high molecular weights, most of which are polymers.
Polymers
Molecules made of a repetitive series of identical or similar subunits called monomers.
Monomers
The identical or different subunits that make up polymers.
Dehydration synthesis
The process of covalently linking monomers together by removing a hydroxyl (-OH) group from one monomer and a hydrogen (-H) from another, producing water as a by-product.
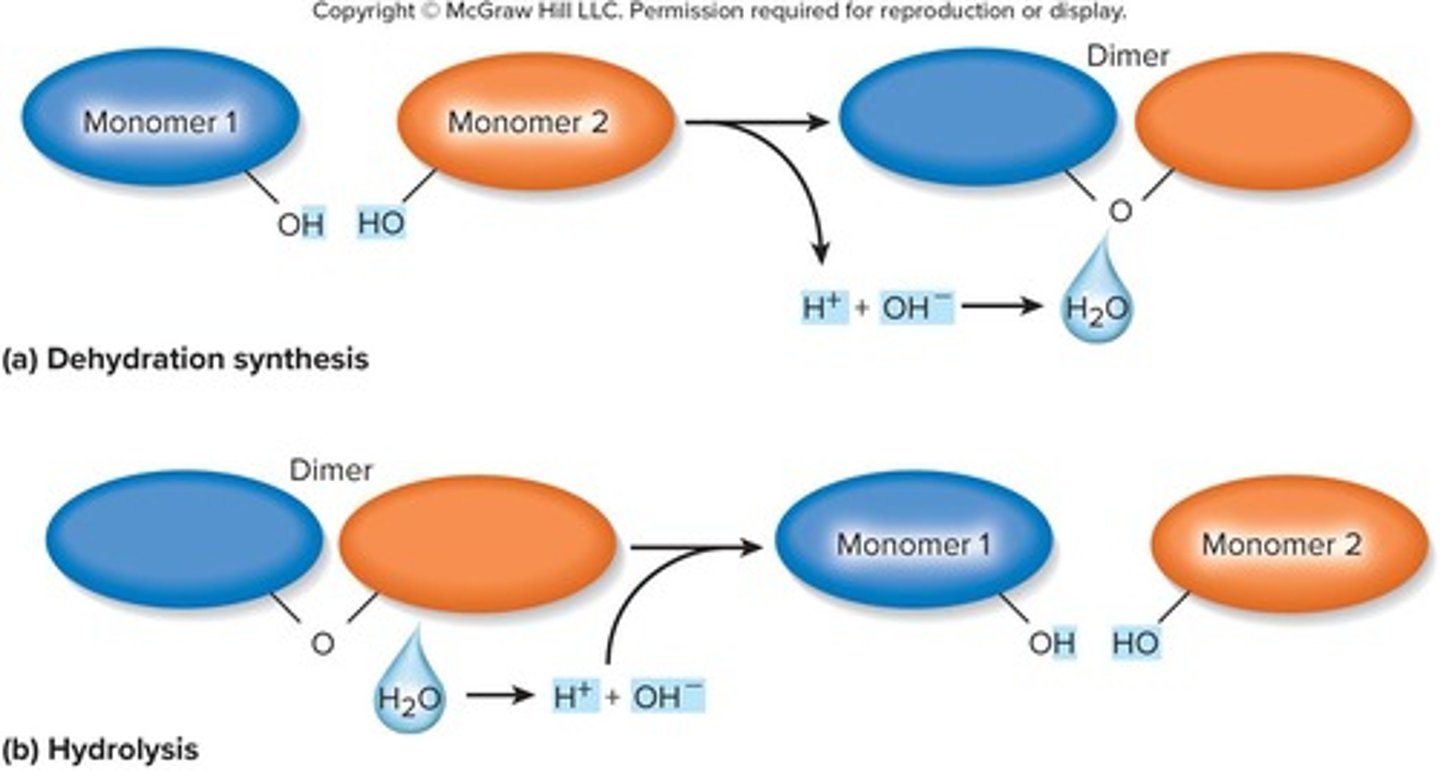
Hydrolysis
The process of splitting a polymer into monomers by the addition of water.