Chapter 6 Materials
1/89
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
90 Terms
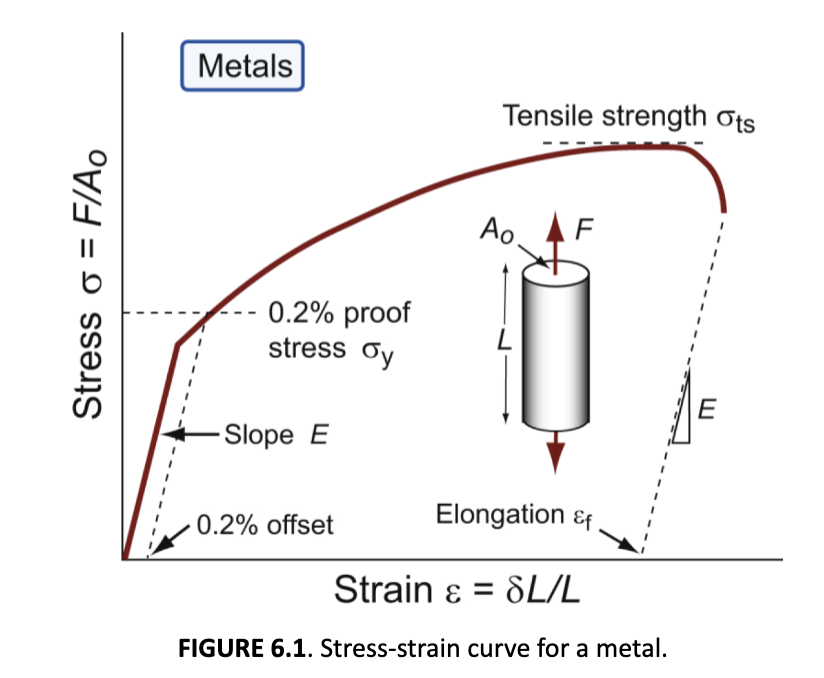
yield strength
the stress beyond which a material becomes plastic; yielding is both useful (forming, absorbing energy) and dangerous
0.2% proof (or offset yield) stress
The yield stress, σy (or elastic limit, σel), requires careful definition. In metals, the onset of plasticity is not always distinct. We will define:
tensile strength
Beyond the yield point, most metals work harden, causing the rise to a maximum
necking
after a material reaches its tensile strength, it undergoes non-uniform deformation (this) and fracture; relatively large amounts of strain localize disproportionately in a small region of the material. resulting prominent decrease in local cross-sectional area. decrease in stress, but increase in strength
ducility
measured by the amount of elongation to fracture - how much you can deform an object before it breaks
failure
yield properties and ductility are measured with standard tensile tests continued to _________;
line: elastic
curve: plastic
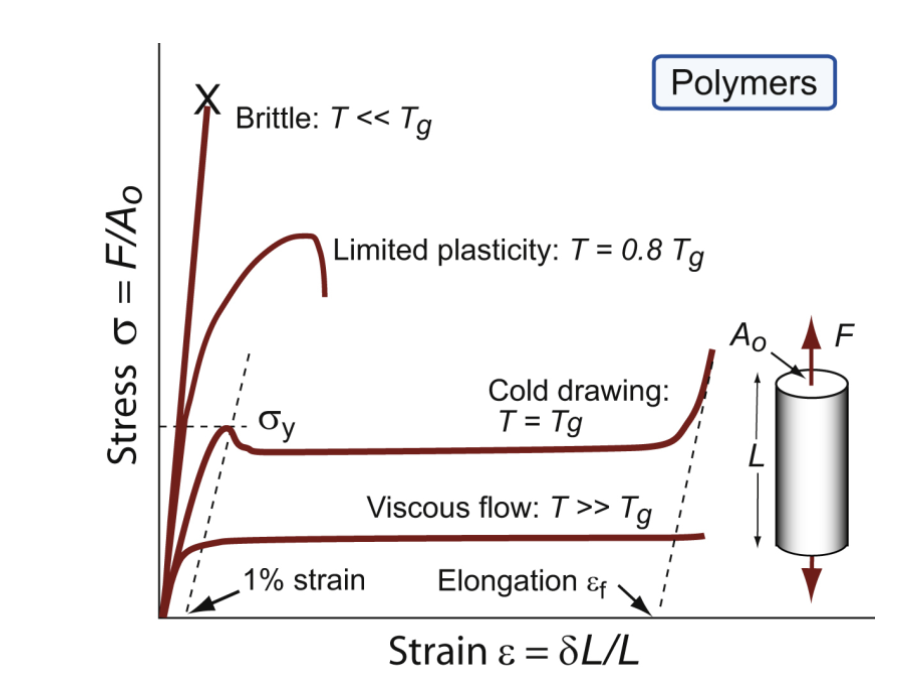
nonlinear; temperature
for polymers, σy is the stress at which the stress-strain curve becomes markedly _________; typically, a strain of 1% - beyond, yielding behavior depends on ________-
brittle polymer
well below Tg
some plasticity in polymer
approaching Tg
cold drawing
polymers at Tg ; large extension at constant stress, molecules gradually align, harden, and then fracture. Neck forms that is stronger than the rest of the material, and more and more joins the neck
viscous flow
at higher temperatures, polymers become a liquid with __________; moldable; thermosets instead become rubbery and then decompose
set deviation of 0.5%
σy for a polymer matrix composite is defined by this
plastic deformation in thermoplastics
dependent on temperature; high temp breaks the intermolecular bonds easily
plastic strain
εpl is the permanent strain resulting from plasticity, the total minus the recoverable elastic part:
εpl = εtot - εel = εtot - σ/E
ductility
measure of how much plastic strain can be tolerated; in a standard tensile test, it is measured by the elongation εf (tensile strain at fracture in %). Strictly speaking, it depends on sample dimensions and is not a material property.
plastic work
done in deforming a material permanently by yielding or crushing. The increment done for a small permanent extension or compression dL under force F per unit volume V=AoLo is
dWpl = F dL/V = F dL/(AoLo) = (F/Ao)(dL/Lo) = σdεpl
Thus
Wpl = ∫ from 0 to εf (σdεpl)
area under stress-strain curve (including plastic deformation region)
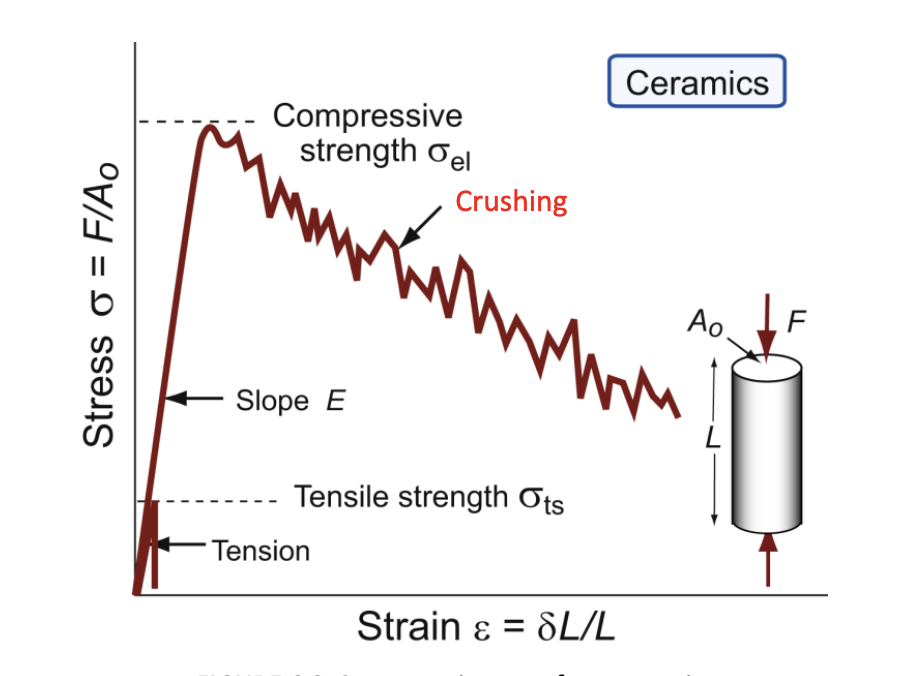
ceramics and glasses
brittle at room temperature- they have yield strengths, but these are never reached because they fracture first; even in compression, they crush before they yield
compressive crushing strength
sometimes this practical measure is used; not a true yield strength, but is called the elastic limit σel
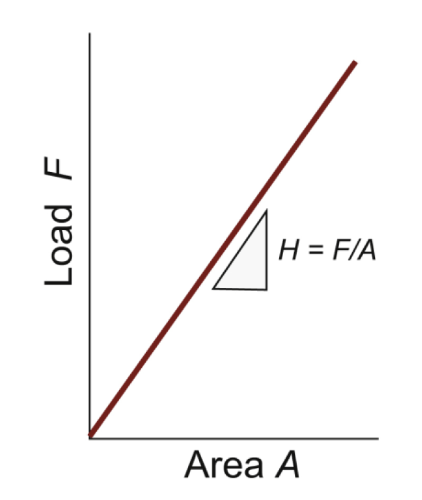
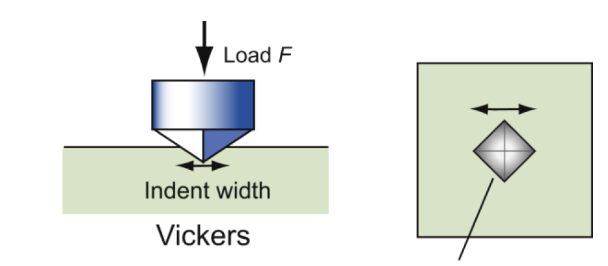
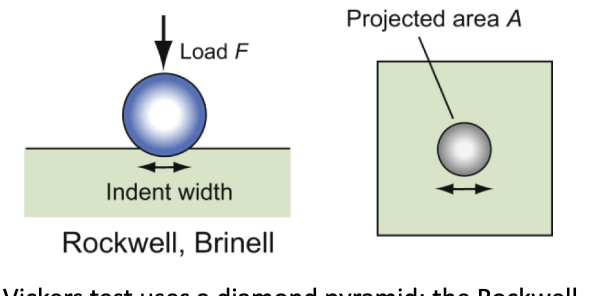
hardness test
an indenter is pressed into surface; the size of the permanent indentation is a measure of resistance to plastic deformation.
Easier and more or less nondestructive. Nonlinear scales often used
Vickers hardness
Brinell hardness
hardness
H = F/A; A is the area of the indentation projected onto a plane perpendicular to the load. The indented region is constrained by surrounding material and thus H > σy
Nonlinear scales often used
narrow range; manipulable
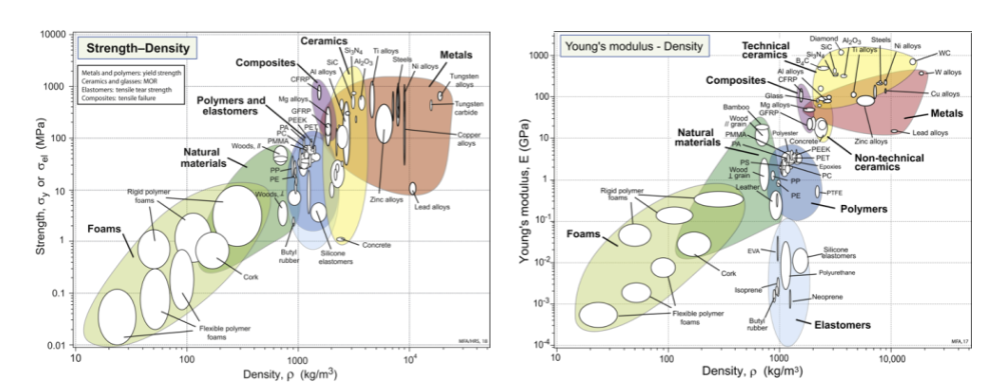
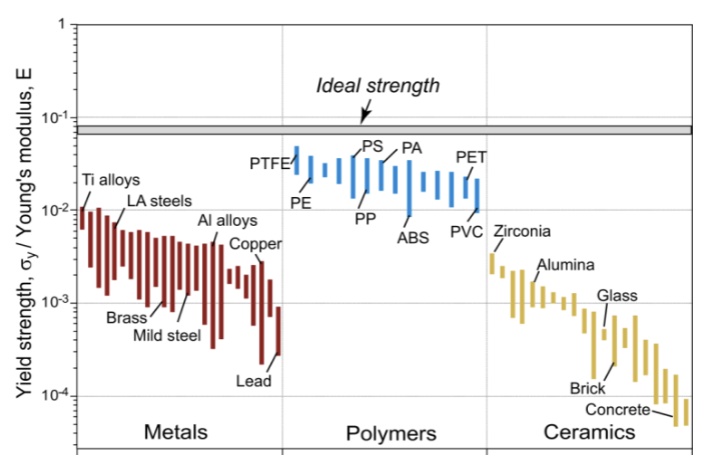
the elastic modulus typically has a _________, while strength can span a decade or more - it is ___________
two major differences between E-density chart and strength density chart
elastomers → low E, amorphous, glass transition temperature is really low, behaving like a liquid at room temp, molecules cross link, brittle at low temp, away from other materials; strength: pull until it snaps, moves to same correlation with other materials
bubbles are small in metals for E vs density, not for strength - large changes in vertical direction because you can adjust strength by different processing methods
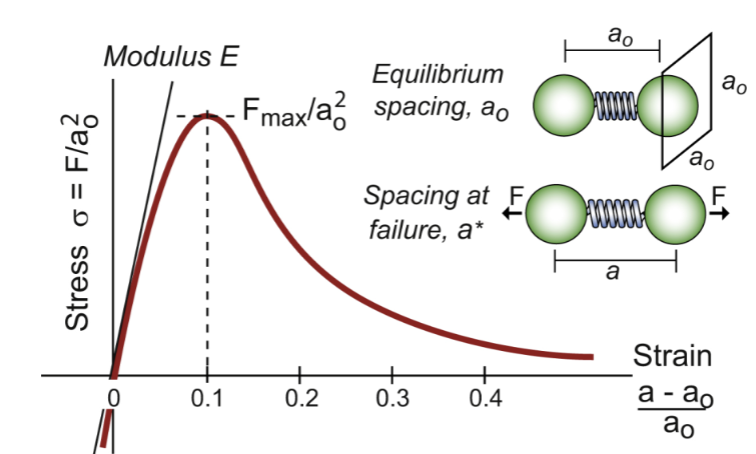
bonds
like springs, have a stress-strain curve; straight line slope is E. Fmax/ao² is ideal strength of the material - drops as atoms pull apart. Resists more when squeezing vs pulling
bond stress
approximate σ=F/a0², stretch from initial length a0 to a, giving a strain (a-a0)/a0
bond strength
peak strength on stress-strain curve for atomic/molecular bonds
broken
the bond is “________” when stretched more than ~10% of the original length (strain ~ 0.1)
S=F/δ and δ=a0/10, so F to do this is ~Sa0/10
ideal strength
should be σideal ~ Fmax/a0² = S/(10a0) = E/10
σideal/E ~ 1/10, more refined analysis gives ~1/15
crystalline imperfection
defects in metals and ceramics; often organized by dimensionality: point defects, dislocations, grain boundaries
real materials
riddled with imperfections (with a few exceptions) and these defects explain strength, diffusion, conductivity, etc
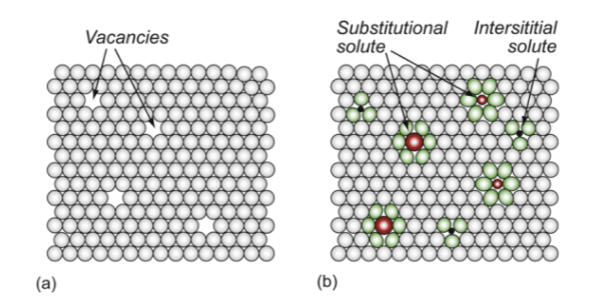
point defects
vacancies, impurities - substitutional, interstitial; 0 dimensional defect
missing atoms; or foreign (solute) atoms in interstitial and substitutional sites (introduces stresses due to difference in sizes)
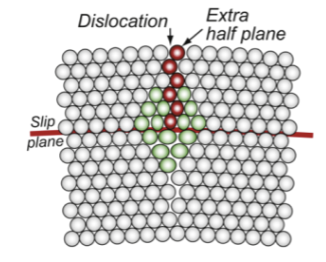
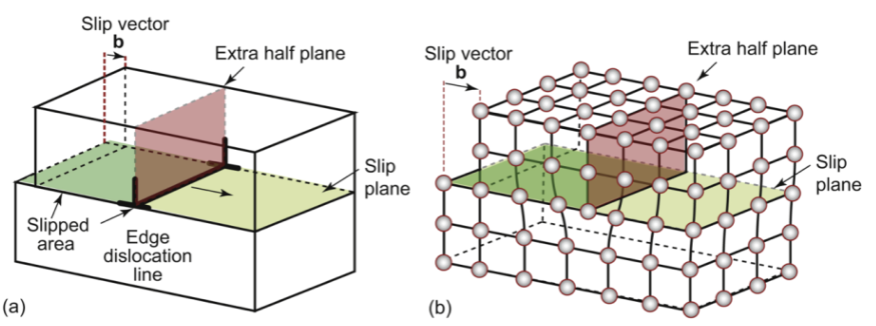
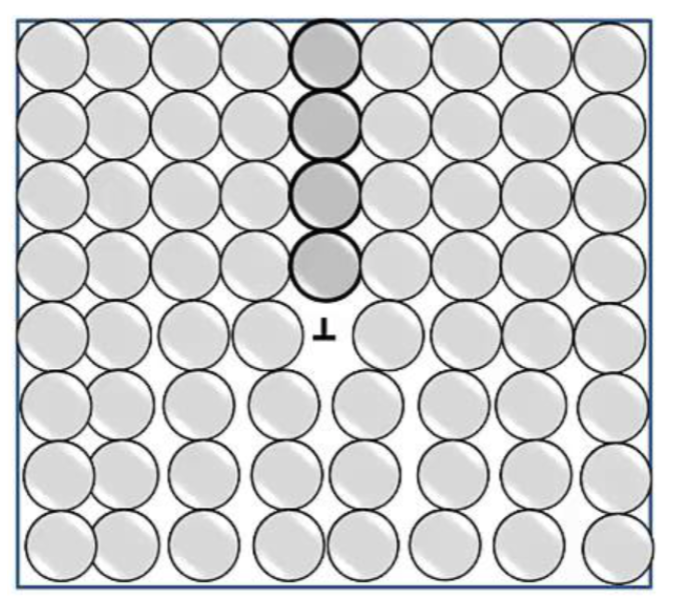
dislocation
an extra half-plane of atoms; can move
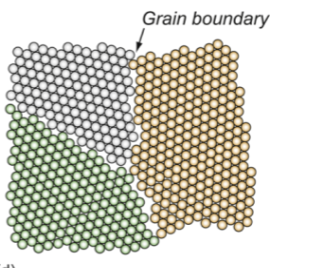
grain boundary
planar (2D) defect on the surface of a material; occurs between any two crystals with slightly different orientations - atoms don’t match exactly, causing distortion
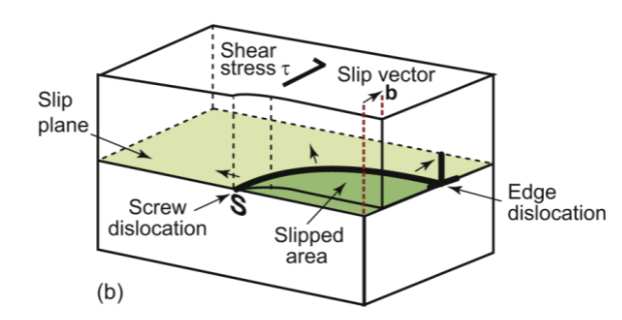
edge dislocation
edge of extra “half-plane.” The dislocation line separates the part of the plane that has slipped from the part that has not. Movement of atoms!
Introduces a shear stress that shifts the dimensions by cutting, slipping, and rejoining bonds across a slip plane. The slip vector b is perpendicular to the edge dislocation line.
compressive and tensile
edge dislocations cause __________ stress where there is an extra half plane, and __________ stress where there is the normal arrangement of atoms
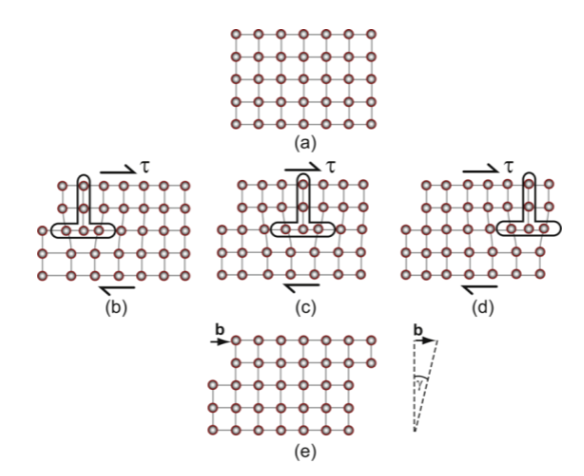
slip vector
Burger’s vector b describes the direction and magnitude of the relative motion and results in a shear strain γ at the end of the process. Upper half moves relative to the lower half by one dislocation (vector b)
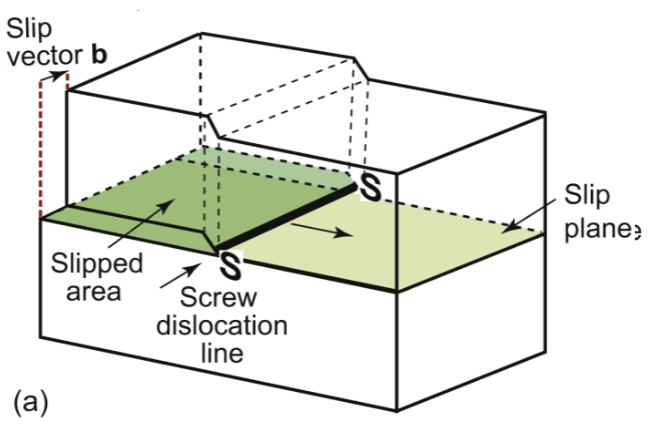
screw dislocation
displacement parallel to the edge of the cut, rather than perpendicular. Geometry is similar to a corkscrew. Like a spiral ramp; has a shear stress along its length. moves backward by one atomic spacing
mixed dislocations
made up of little segments of both edge and screw - b is fixed, while the line wanders. It is far easier to move a dislocation through a crystal, breaking and reforming bonds along a line, than simultaneously breaking all of the bonds in an entire plane. it can be likened to moving a carpet a short distance by pushing a small fold across. These are not in straight lines!
slip systems
Dislocations move more easily on some planes and in some directions than others — preferred slip planes, slip directions; depend on crystal structure
many dislocations
individual slip displacements are tiny; with _____________ moving on many planes, macroscopic shape changes can occur, while volume is essentially unchanged
FCC slip systems
close-packed planes are perpendicular to body diagonals of a cube — 4 planes per unit cell
Atoms in close-packed layers touch along face diagonals — 3 directions per close-packed plane
→ 12 slip systems
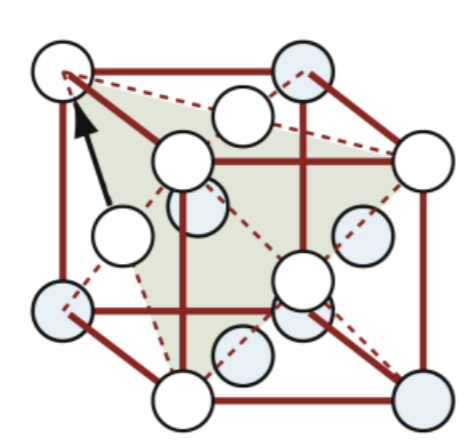
very ductile when pure
FCC metals are usually…
crystallography
controls strength and ductility of pure metals
friction-like resistance
crystals resist dislocation motion with this f per unit length - yielding occurs when an external force (Fs) exceeds f. Breaking bonds requires energy
static until it is exceeded
line tension
dislocations attempt to keep their length as short as possible - they behave as if they have this, T, like a rubber band; T ~ Eb²/2 - can be lowered by reducing b (in close-packed planes in FCC metals)
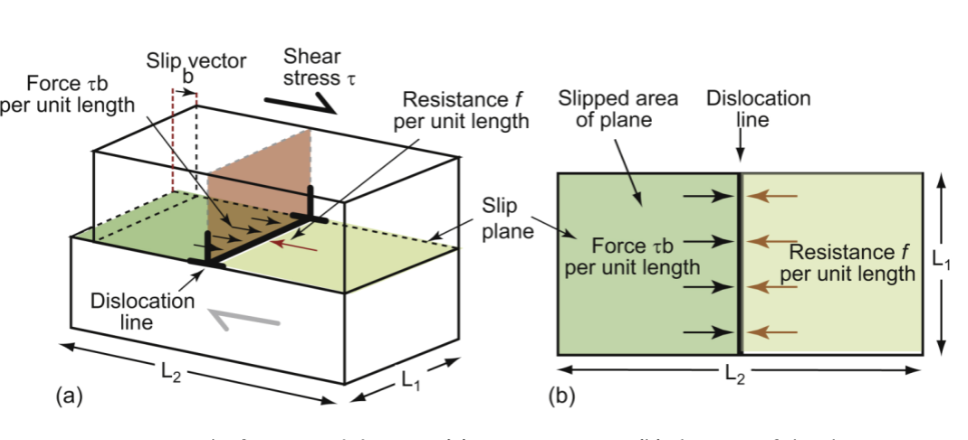
lattice resistance
contributions to f: fi (the intrinsic resistance of the lattice to shear due to bonding) is lower in metals, but very high in ceramics. however, metals can be further strengthened, while ceramics are prone to fracture
obstacles to slip in metals and alloys
solid solution hardening, fss, precipitation hardening, fppt, working hardening via new dislocations, fwh, and grain boundaries, fgb.
can be manipulated in manufacturing
perfect lattice
resistance fi
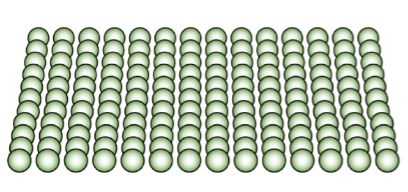
solution hardening
resistance fss, adding specific atoms to the crystal (alloying) or impurities
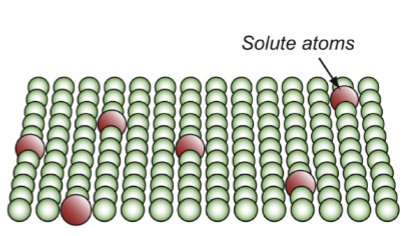
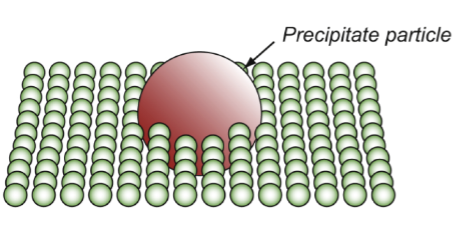
precipitate hardening
resistance fppt
other particles/crystals in the lattice that are bigger than the atoms
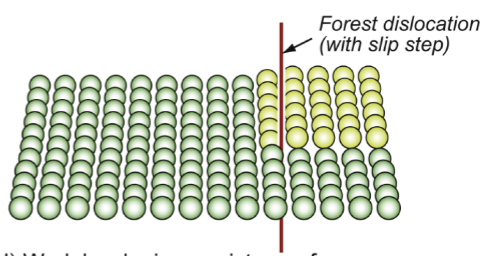
work hardening
resistance fwh
add new defects into the crystal; parallel, can repel one another
total shear yield strength
is the sum of the intrinsic shear strength of the lattice plus the contributions due to solid solution hardening, precipitate hardening, work hardening, and grain boundary hardening. Need to extend from loading in shear to loading in tension
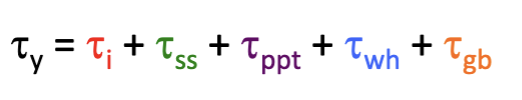
impede dislocation motion
to strengthen metals, obstacles increase f and improve strength - found from spacing and strength of interaction; the shear stress needed to force the dislocation through the field of obstacles has the form Δτ = αEb/L
where α is dimensionless and characterizes the obstacle strength of interaction with a dislocation (between 0 and 1)
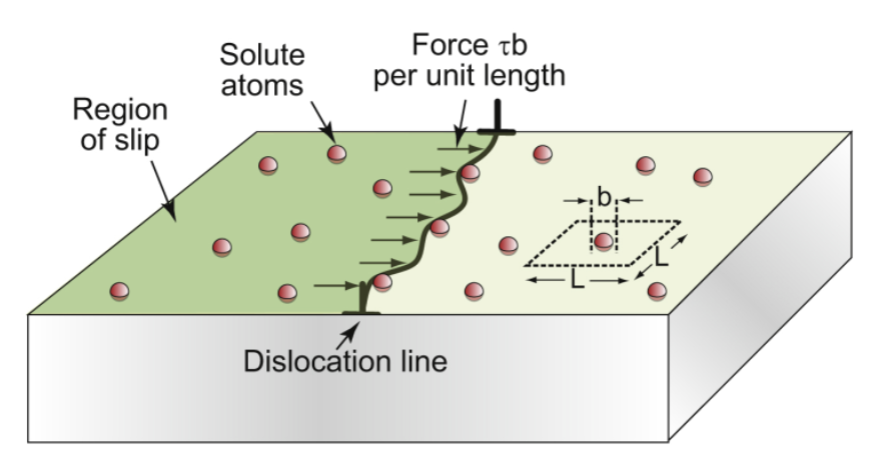
solution hardening
deliberate addition of impurities (alloying). Every element has a slightly different size; this locally strains the lattice, which “roughens” the plane and interferes with dislocation motion
concentration (atom fraction)
c = b²/L² thus L=b/c1/2
this gives τss= αEc1/2
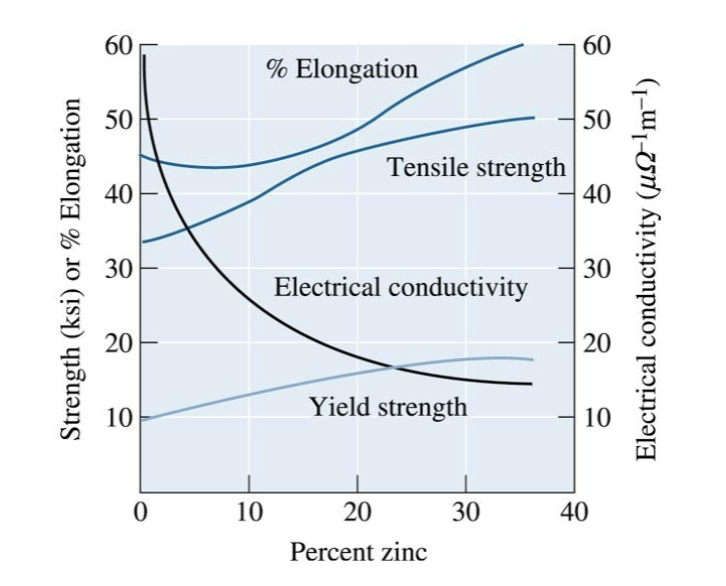
atomic size difference and amount of alloying element
nickel and zinc atoms are about the same size as copper atoms, but beryllium and tin have greater effect on yield strength because their larger difference in size causes a bigger hindrance to movement of dislocation, which increases strength.
Increasing both _____________ and ____________ increases solid-solution strengthening
solid solubility
liquid copper and liquid nickel are completely soluble in each other. Solid copper-nickel allows display complete ___________, with copper and nickel atoms occupying random lattice sites in the FCC crystal.
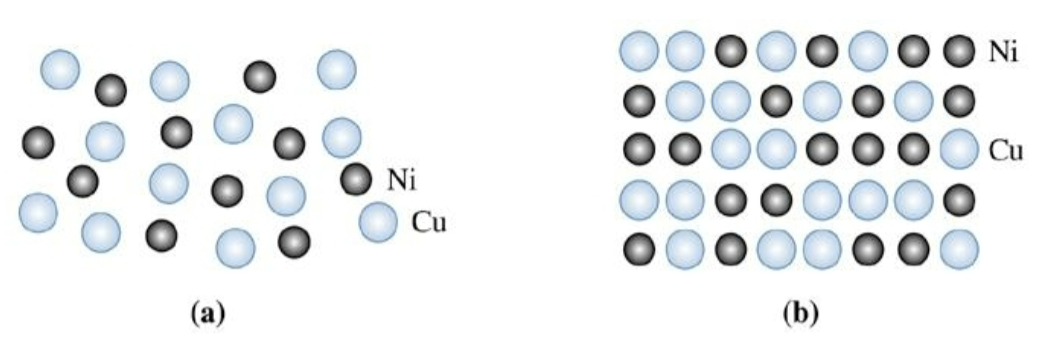
second phase; limited solubility
in copper-zinc allows containing more than 30% Zn, a ___________ forms because of the ______________ of zinc in copper. Exceeding this value means a precipitate forms
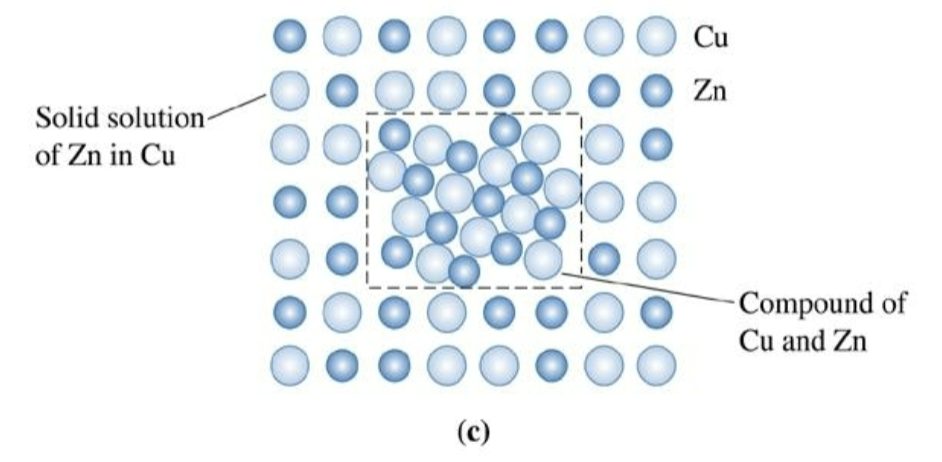
phase
a structurally homogeneous (same crystal structure) and distinct portion of matter (has boundaries surrounding it that separates it from others).
solubility limit
in metals, is mainly a function of relative atomic size. If two metals have atoms that differ widely in size, they will not be very soluble in one another
dispersion and precipitate strengthening
dispersed small, strong particles either in a metal matrix composite (by mixing) or via an in-situ precipitation process (exceeding the solubility limit). Spacing between atoms is the critical factor
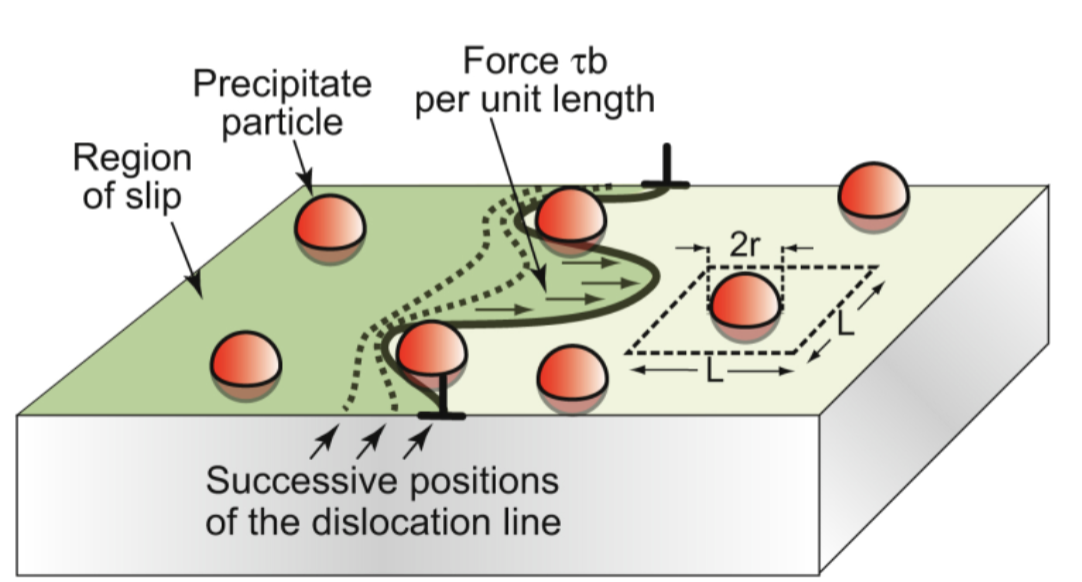
dislocation pinning
a. force on dislocation between pinning points
b. dislocation bowing and escape from weak obstacles
c. dislocation escape strong obstacles
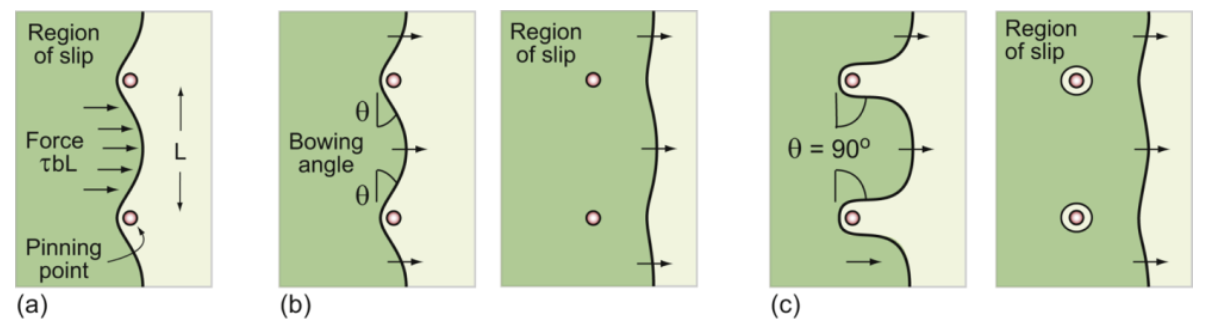
dislocation bows
between particles against line tension; wants to be as short as possible
critical configuartion
of precipitate strengthening, the semicircle where the total force on a line segment of length L is just balanced by the force 2T due to line tension
dislocation escapes
when τppt = 2T/bL ~ Eb/L
work hardening
add more dislocations to the material to increase the strength; dislocations are generated by plastic deformation
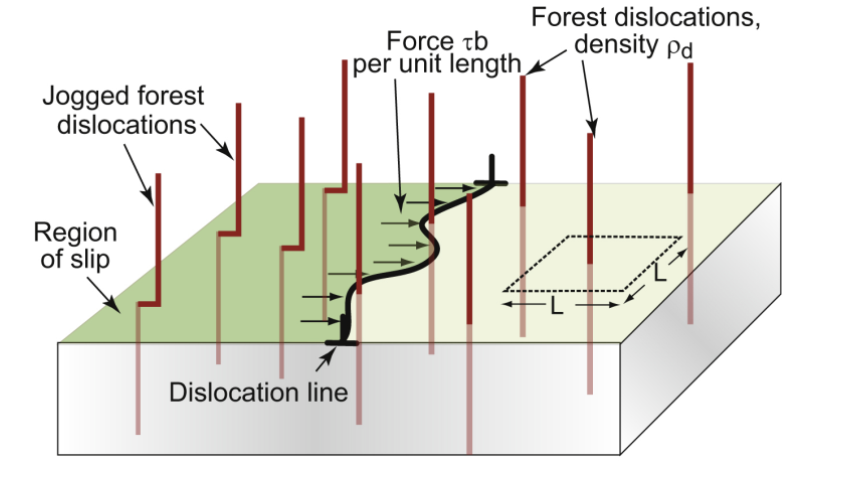
dislocation density
the line length per unit volume; even a well annealed sample has this value in the 10^10, deformed can be 10^17
forest; job
in work hardening, a moving dislocation runs into a “________” of dislocations with average spacing L=ρd-1/2 and creates a “____” of length b in each other dislocation, each exerting a pinning force of p=Eb²/2
yields τwh ~ Eb(ρd-1/2)/2
strengthening by deformation
in metals, is related to increasing the dislocation density; at stresses higher than the yield strength, dislocations begin to move (called slip) and then encounter obstacles (pinning. Even higher stresses produce more dislocation → leads to more interaction, more entanglement, and more strengthening
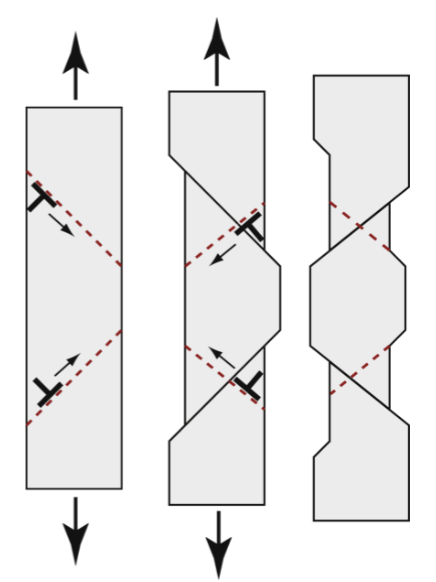
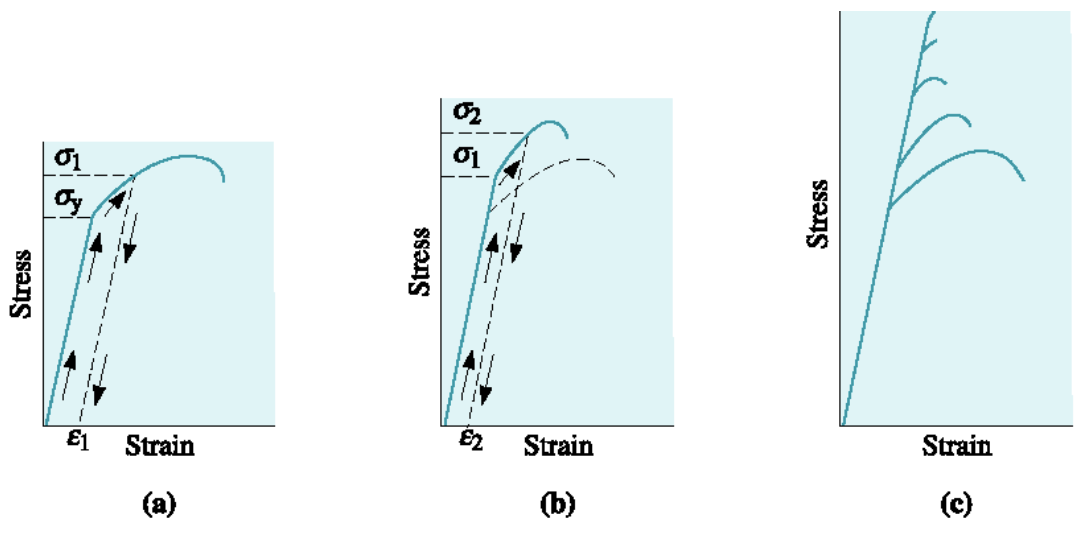
cold work
repeated application of loads beyond the yield strength increases the yield and tensile strengths, but decreases ductility until the yield, tensile, and breaking strengths coincide - then, no further plastic deformation is possible.
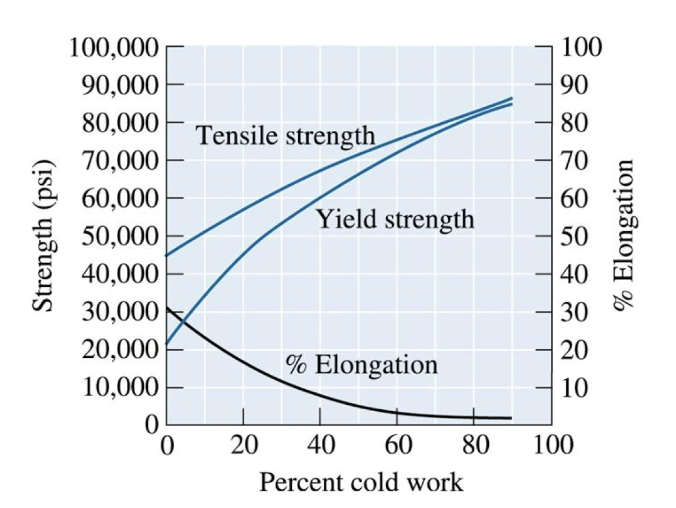
percent cold work
%CW = 100 x (Ao - Af)/Ao
Ao is original cross-sectional area
Af is final cross-sectional area.
As this increases, the yield strength and tensile strength increase, but ductility decreases.
crack
there is a maximum amount of cold work that a material can accommodate. If more cold work than this is attempted, the material will…
final steps in processing
mechanical behavior requirements can be met through design of the deformation process. one of … can be plastic deformation to get the required final strength
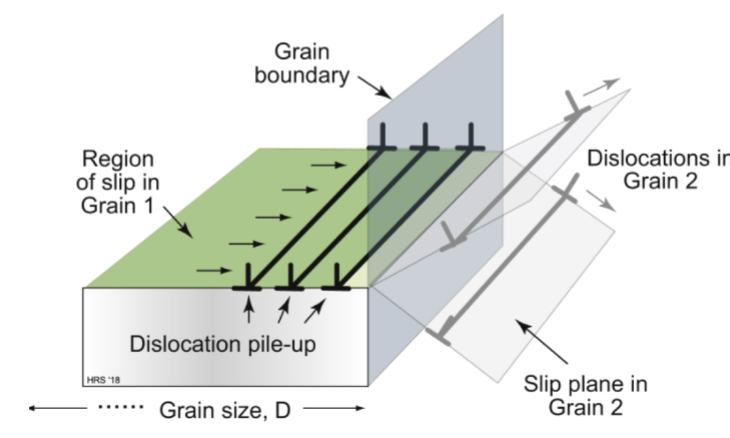
grain boundary hardening
these disrupt dislocation motion; experimentally determined relationship is τgb ~ kp/D1/2
where kp is the Petch constant and D is grain size. The effect increases significantly when grains approach 1 micrometer or smaller
grain boundaries
many materials are polycrystalline (made up of many grains); each grain has the same crystal structure, but is oriented differently in space from other grains
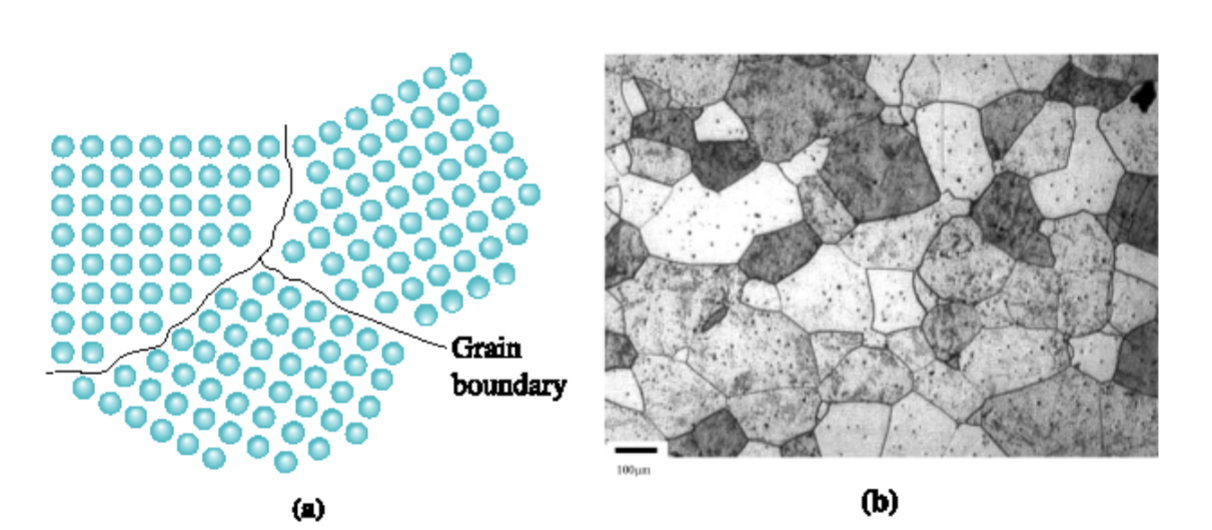
boundaries; interiors
the atoms in the __________ between grains are not spaced and positioned as they are in the _________ of the grains; no equilibrium spacing
acid solution on surface
energy is higher in the grain boundary → preferentially attacked and make grooves in the metal sample to estimate the grain size
impede dislocation motion
grain size impacts mechanical behavior because grain boundaries…
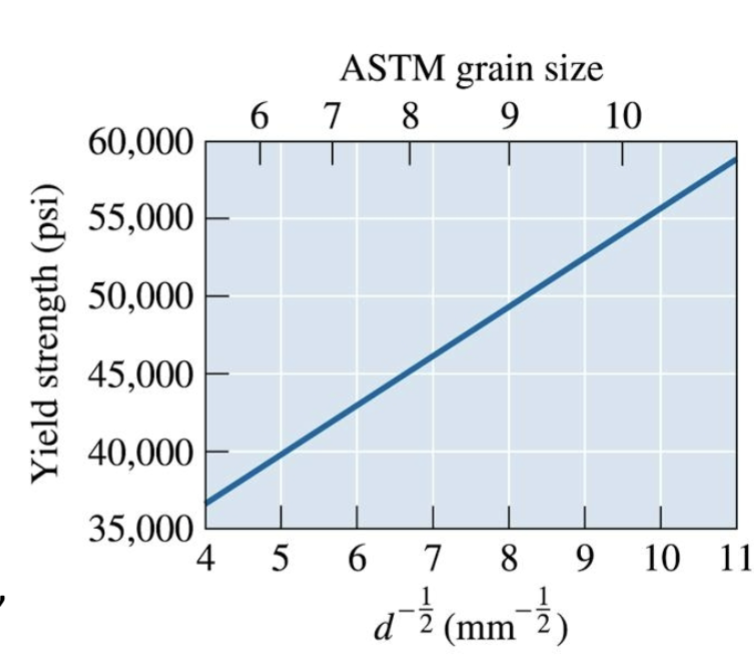
Hall-Petch equation
relates grain size to yield strength: σy = σo + Kd-1/2
σy is the yield strength; d is the average grain diameter, and σo and K are constants
total shear yield strength
to a first approximation:
τy = τi + τss + τppt + τwh + τgb
= τi + αEc1/2 + Eb/L + Eb(ρd)1/2 /2 + kp/(D)1/2
related to spacing of obstacles
optimize properties
Manipulating the strength of metals has been the most explored area of materials science; hardening mechanisms are often combined to…
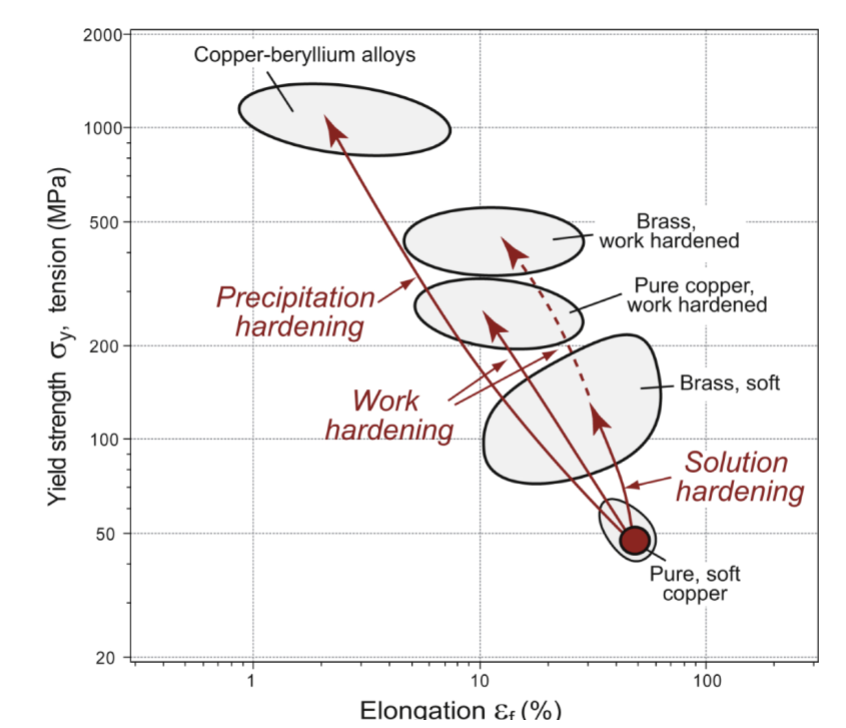
lower ductility
increased strength leads to …
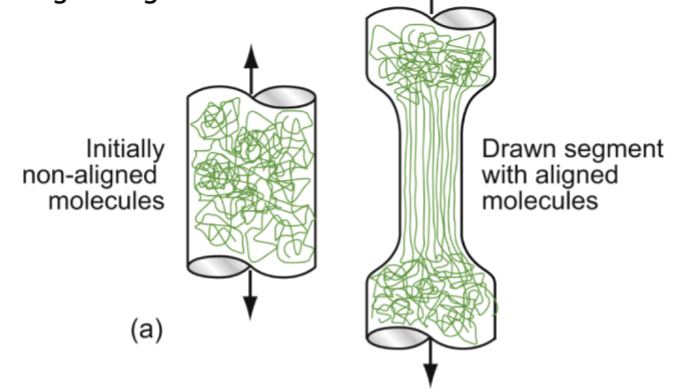
drawing
above ~0.75Tg, thermoplastic polymers exhibit some ductility. During _______, the chains slide over each other, unravel, and align (in 1 or 2 dimensions, no 3D). The material is now stiffer and stronger than before, but possible geometries are limited.
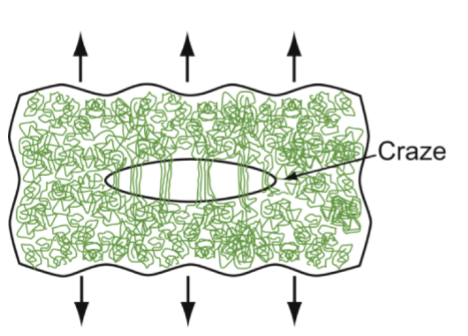
crazing
PE and PP draw at room temp; PMMA requires higher T. Drawing at too low a temperature leads to ________ - incipient cracks with ligaments bridging the craze surfaces - if continued, it fractures
strengthening of polymers
dislocations are not relevant, there is relative slippage of polymer chain segments or shear among molecular clusters in a glass.
impeding the slippage
strengthening in polymers is achieved by doing this via blending, drawing, crosslinking, and reinforcement; breaking and reforming of bonds
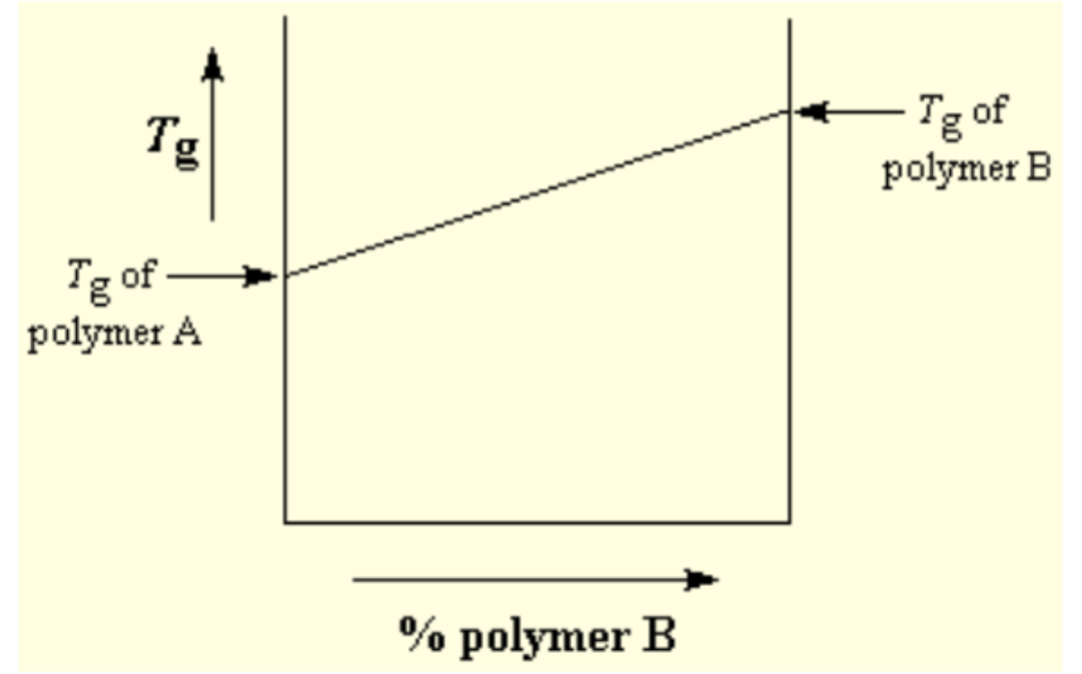
blend
mixture of two polymers; property variation can be linear or non-linear
drawing
alignment by stretching (mylar film, fishing line)
crosslinking
replace weak Van der Waals bonds with strong covalent bonds; vulcanized rubber, epoxies; make more rigid
reinforcement
add cheap fillers or engineered fibers to create a composite material