Atomic structure
1/7
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
What idea did Robert Boyle propose about the atom?
That there were some substances that could not be made simpler called chemical elements.
What idea did John Dalton propose about the atom?
That elements were composed of indivisible atoms. All the atoms of a particular element had the same mass and atoms of different elements had different masses. Atoms could not be broken down.
What did Henri Becquerel discover about the atom?
Henry Becquerel discovered radioactivity, which showed particles could come from inside the atom, and therefore not divisible.
What idea did JJ Thomson propose about the atom?
JJ Thomson discovered the electron. He showed that electrons are negatively charged and electrons from all elements were the same. Therefore suggesting the plum pudding model.
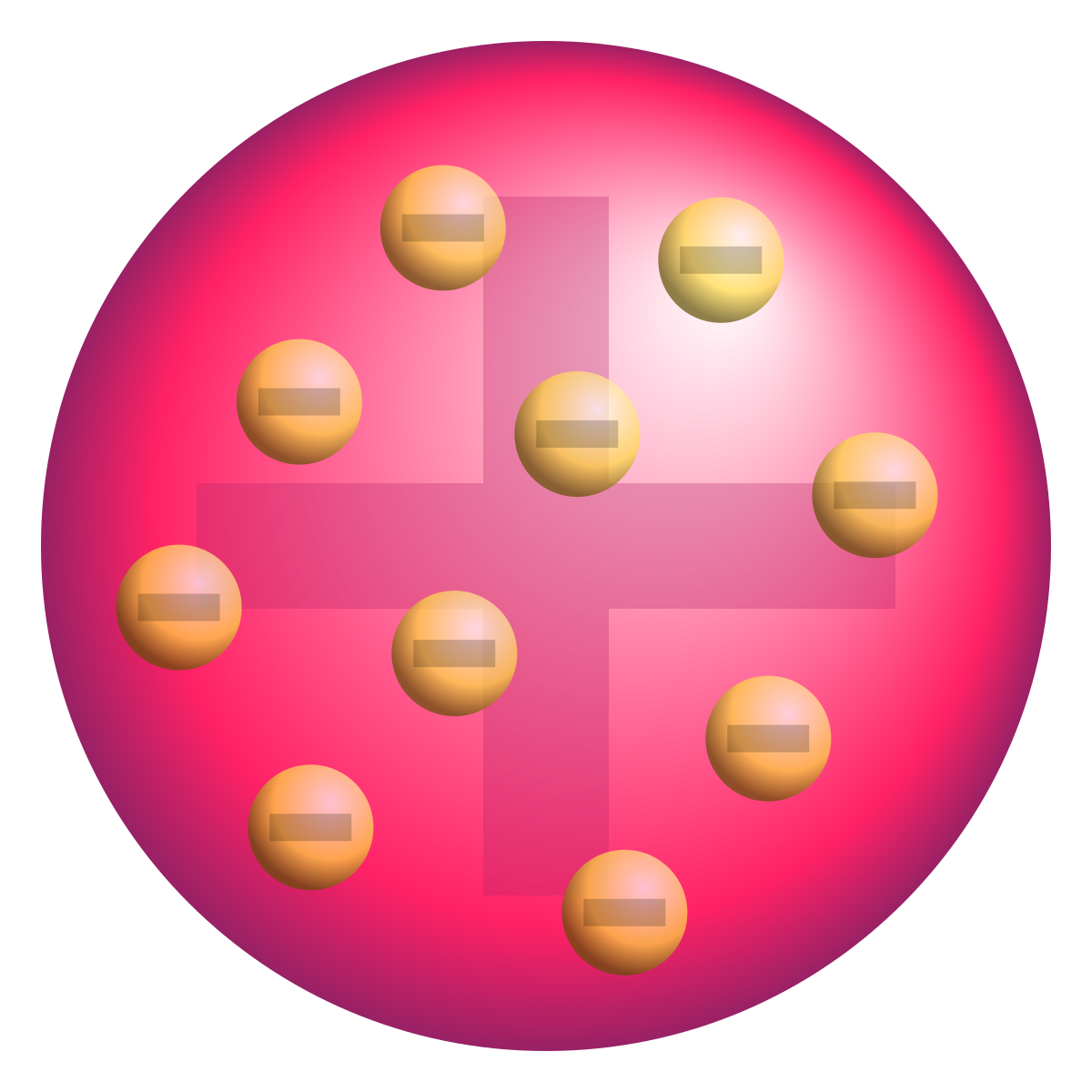
What idea did Ernst Rutherford discover about the atom?
Most of the mass, and all the positive charge of the atom was in a tiny central nucleus.
Name all 3 sub-atomic particles
Proton
Electron
Neutron
Define nucleons
The sub-atomic particles found in the nuclei of atoms. E.g. protons and neutrons.