Chapter 6
1/313
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
314 Terms
What do we mean when we say that enzymes are catalysts?
They increase reaction rates without being used up.
What kind of proteins are enzymes?
Globular.
Other than globular proteins, what can also catalyze reactions?
RNA (ribozymes and ribosomal RNA)
What is the oldest field of biochemistry?
Study of enzymatic processes (late 1700s)
Enzyme History: Buchner

Enzyme History: Sumner
Enzyme History: Haldane
1930’s suggested weak bonding interactions between the enzyme and substrate might be used to catalyze a reaction
catalytic activity depends on the
integrity of the native protein conformation
Molecular weight of enzymes =
ranges from 12,000 to >1 million
cofactor =
1+ inorganic ions, such as Fe2+, Mg2+ , Mn2+, or Zn2+
coenzyme =
complex organic or metalloorganic molecule that act as transient carriers of specific functional groups
Structure essential to
catalytic activity
Inorganic ions:
Inorganic ions:
Coenzyme: ________or __________ • Prosthetic group: _____ ______• HOLOENZYME = _____+ ______• Apoenzyme: ________ _____
Organic or organometallic; tightly bound; enzyme + cofactor; missing cofactor
What is a cofactor?
An inorganic ion or coenzyme required for enzyme activity.
What is a prosthetic group on an enzyme?
A coenzyme or metal ion tightly or covalently bound to it’s protein
What is a holoenzyme?
Complete catalytically active enzyme together with its bound coenzyme and/or metal ions
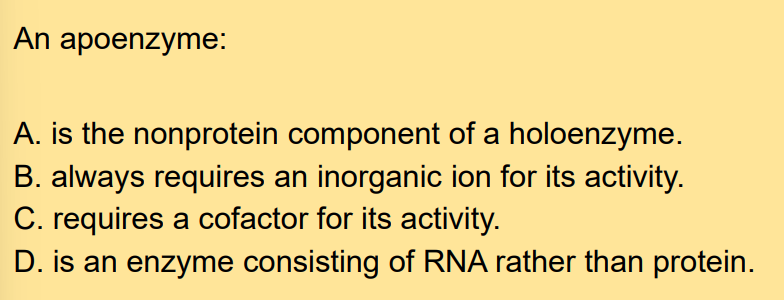
What is an apoenzyme?
The protein part of a holoenzyme.

How are enzymes classified?
By reaction type. with subclasses and formal systematic name vs. common name.
In classifications of enzymes, each has a ….
four part Enzyme Commission number (EC number) and a systematic name.
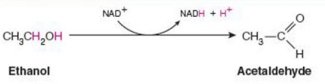
Give Reaction Type and Class
Oxidoreductase.
Alcohol dehydrogenase (oxidation with NAD+)
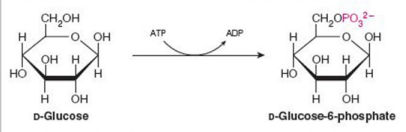
Give Reaction Type and Class
Transferases
Hexokinase (phosphorylation
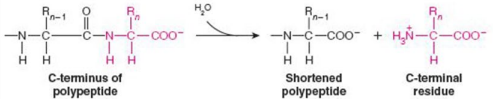
Give Reaction Type and Class
Hydrolases
Carboxypeptidase A (peptide bond cleavage)
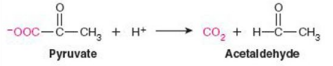
Give Reaction Type and Class
Lyases
Pyruvate decarboxylase (decarboxylation)
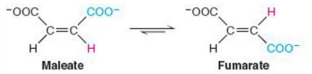
Give Reaction Type and Class
Isomerases
Maleate isomerase (cis-trans isomerization)
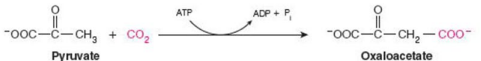
Give Reaction Type and Class
Ligases
Pyrucate carboxylase (carboxylation)


Enzymatic reactions occur in ______.
Specialized pockets called active sites.
What occurs at active sites?
A reaction - the conversion of a substrate to a product. W
What is a substrate?
A molecule acted on by an enzyme.
By how much do enzymes enhance rate?
By 105 to 1017
Enzymes are characterized by the formation of which complex?
Enzyme-substrate
Do enzymes lower the activation barrier?
Yes
Do enzymes affect the equilibrium?
No
What are five reasons why biocatalysis is better than inorganic catalysis?
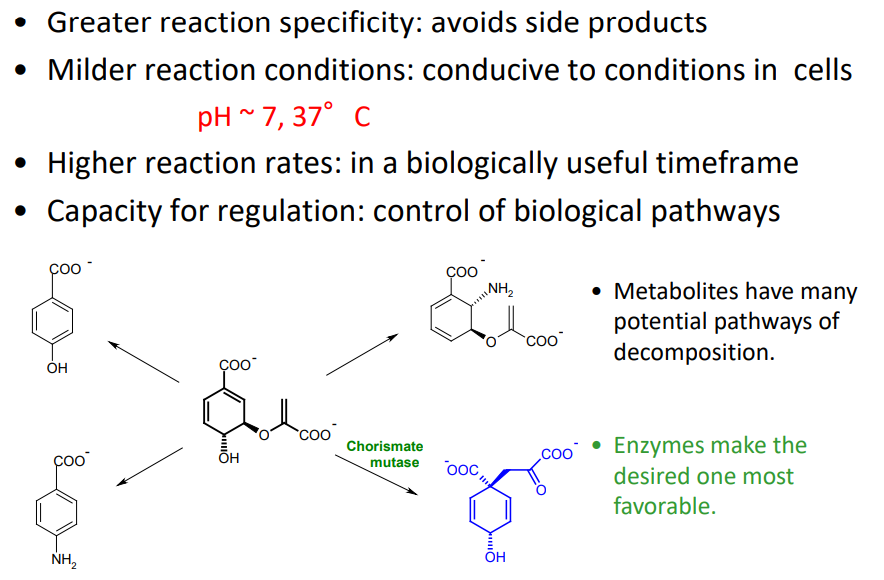
What is Phenylalanine Hydroxylase?
an enzyme that catalyzes the hydroxylation of the aromatic side-chain of phenylalanine to generate tyrosine
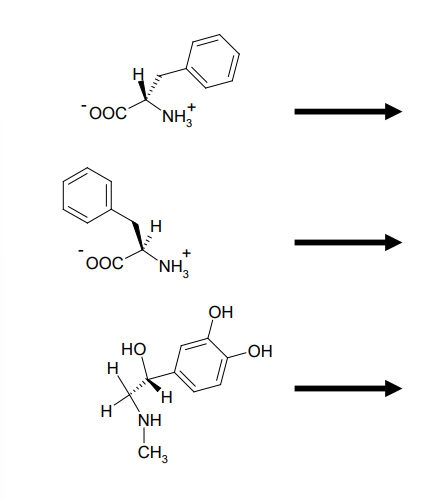
Enzymatic Substrate Selectivity of PAH
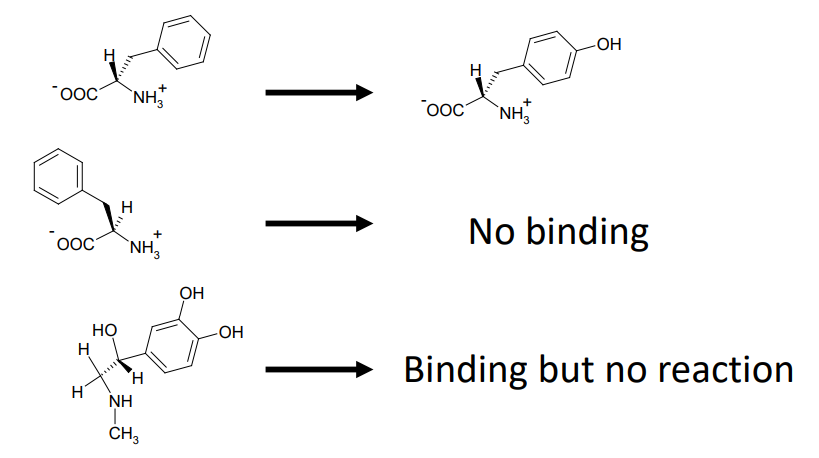
What is an active site?
provides a specific environment in which a given reaction can occur more rapidly
What is a substrate?
the molecule that is bound to the active site and acted upon by the enzyme
simple enzymatic reactions can be written as
where E, S, and P represent the enzyme, substrate, and product and ES and EP are transient complexes of the enzyme
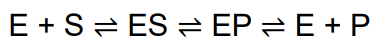
Can enzymes affect the free energy of the reaction?
No
Slow reactions face what to occur?
Activation barriers.
How do enzymes overcome high activation barriers?
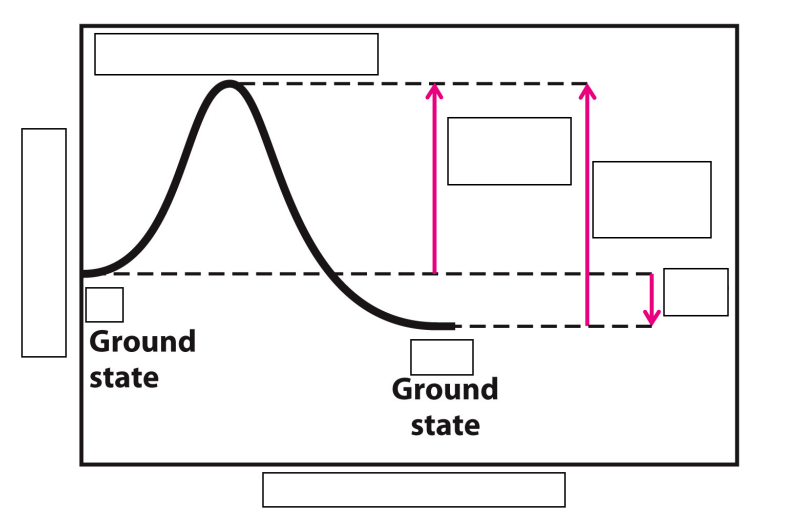
Label this Reaction Coordinate Diagram.
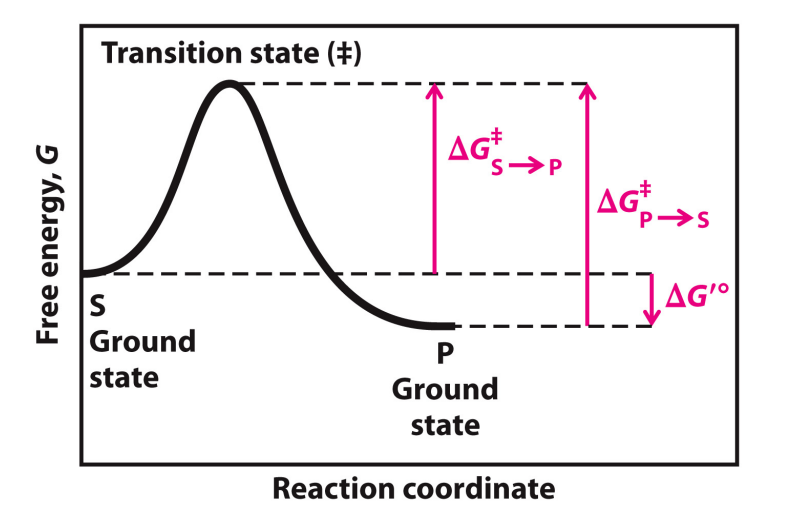
biochemical standard free energy change =

activation energy (∆G‡) =
difference between the ground state energy level and the transition state energy level
any enzyme that catalyzes the reaction S → P also catalyzes the reaction _____.
P → S
enzymes ________the interconversion of S and P
Accelerate
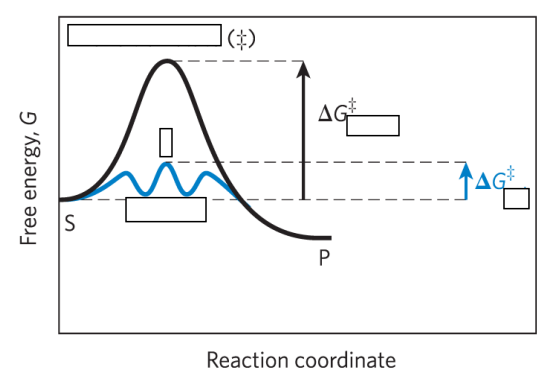
reaction intermediate =
any species on the reaction pathway that has a finite chemical lifetime – example: ES and EP complexes
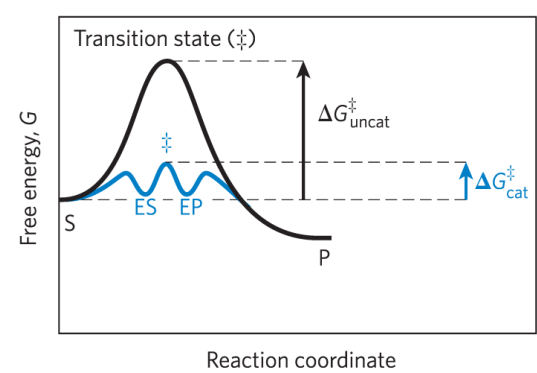
rate-limiting step =
= the step in a reaction with the highest activation energy that determines the overall rate of the reaction


Enzymes exhibit a very high degree of _______
Specificity
Each enzyme catalyzes____ chemical reaction, or sometimes________ reactions
Only one; a few closely related
Reaction activation barriers are thus _____ selectively.
lowered
reaction equilibria are linked to the
standard free-energy change for the reaction, ∆G′°
reaction rates are linked to the
activation energy, ∆G‡
under standard conditions: K′ eq =
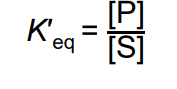
from thermodynamics: ∆G′° =
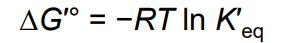
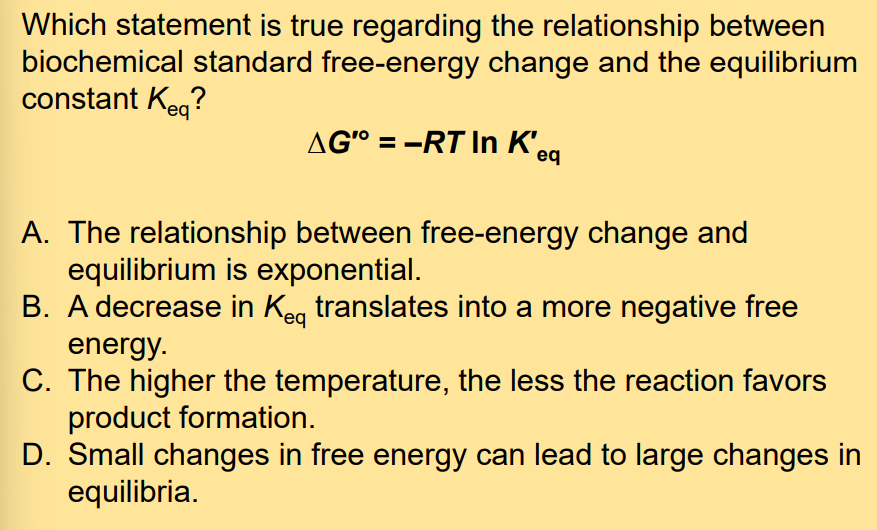
The Relationship between K′ eq and ∆G′ °
Inverse.

the rate of any reaction is determined by the _______________ and the _________
concentration of reactant(s) and the rate constant, k
for the unimolar reaction S → P, a ___________ expresses the rate of the reaction
rate equation

first-order reaction =
rate depends only on the concentration of S
k has units of
reciprocal time, such as s–1


Equation for Relatioinship b/n Rate Constants and Activation Energy
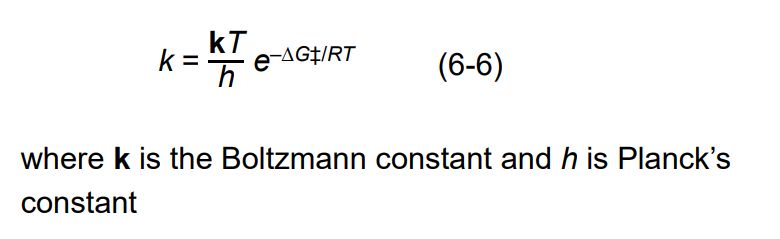
The relationship between the rate constant k and the activation energy ∆G‡ is _______ and ______
inverse and exponential
Boltzmann constant
kB=1.3806 × 10-23 J/K
Planck’s Constant used in the formula for The Relationship between Rate Constants and Activation Energy
h = 6.626 × 10-34 d . s
R for The Relationship between Rate Constants and Activation Energy
R = 8.314 J/ (mol . K)
Enzymes enhance rates in the range of __ to __ orders of magnitude
5 to 17
Rate enhancement =
Carboxypeptidase
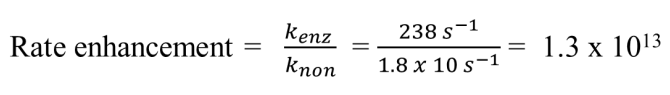
What two concepts explain the catalytic power of enzymes?
Enzymes bind most tightly to the Ts of cat. rxn, using binding E to lower the activation barrier
Enzyme active sites are organized by evo to facilitate multiple mech of chem cat simultaneously.
Define Binding Energy
∆GB = energy derived from noncovalent enzyme-substrate interaction
What interactions mediate binding energy?
H bonds, ionic interactions, and the hydrophobic effect.
What is the major source of free E used by enzymes to lower the activation energy?
Binding Energy
What can covalent interactions bwetween the enzyme and substrate do to the activation energy?
Lower it.
When are noncov int b/n E and S optimized?
In the transition state.
What is the lock and key hypothesis
enzymes are structurally complementary to their substrates – would make for a poor enzyme
What do enzymes bind best? Who proposed this idea?
Transition states. Linus Pauling in 1946
Enzyme active sites are___________ to the transition state of the reaction. Enzymes bind transition states______ than substrates
Complimentary; better
Stronger/additional interactions with the_____ state as compared to the _____ state _____ the activation barrier. Largely___effect
transitional; ground; lower ;ΔH‡
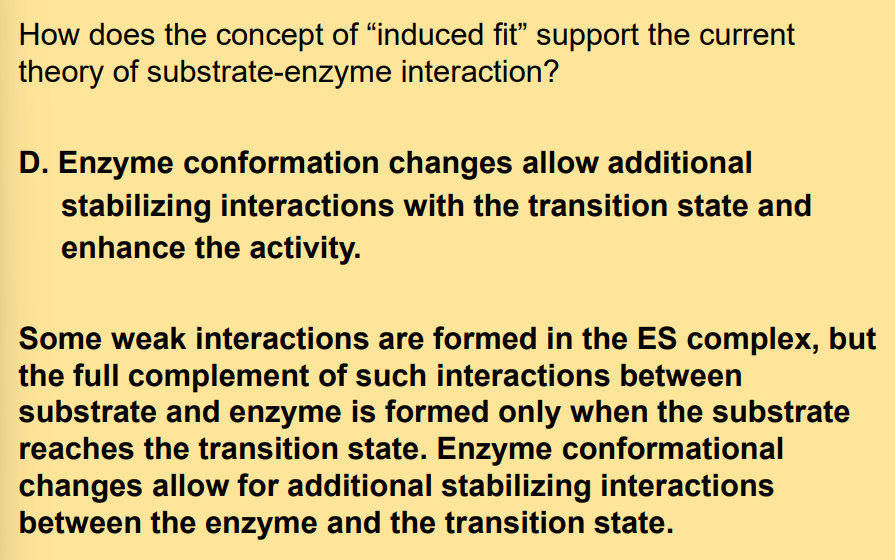
the full complement of interactions between substrate and enzyme is formed only when___________________.
the substrate reaches the transition state
Stickase
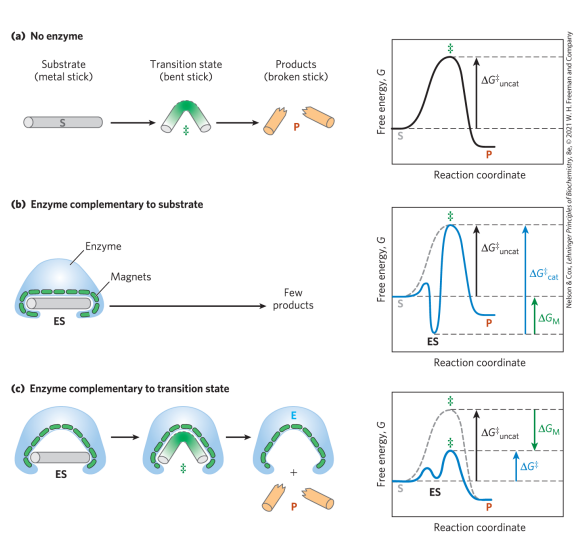
Enzymes must________ the transition state to achieve rate enhancement
preferentially stabilize
the sum of the _____________ and the _____________ results in a lower net activation energy
unfavorable activation energy ∆G‡; favorable binding energy ∆GB
_______________between the enzyme and the substrate drive enzymatic catalysis
weak binding interactions


optimized binding energy in the transition state is accomplished by _____________ (the _______), removed from ___
positioning a substrate in a cavity; active site; H2O
Define specificity. What is it given by?
= ability to discriminate between a substrate and a competing molecule – given by binding energy
barrier to reaction , ∆G‡ includes what four things?
– the entropy of molecules in solution
– the solvation shell of hydrogen-bonded water that surrounds and stabilizes most biomolecules in aqueous solution
– the distortion of substrates that must occur in many reactions
– the need for proper alignment of catalytic functional groups on the enzyme
Enzymes organize reactive groups into _______ and _________
Close proximity and proper orientation
Uncatalyzed bimolecular reactions
two free reactants → single restricted transition state conversion is entropically unfavorable
Uncatalyzed unimolecular reactions
flexible reactant → rigid transition state conversion is entropically unfavorable for flexible reactants
Catalyzed reactions
Enzyme uses the binding energy of substrates to organize the reactants to a fairly rigid ES complex
Entropy cost is paid during binding
Rigid reactant complex → transition state conversion is entropically OK
Entropy Reduction
large restriction in the relative motions of two substrates that are to react
binding energy constrains substrates in the _______ to reaction
proper orientation
Desolvation
replacement of the solvation shell of structured water around the substrate with weak bonds between substrate and enzyme