Resonance Structure Patterns
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
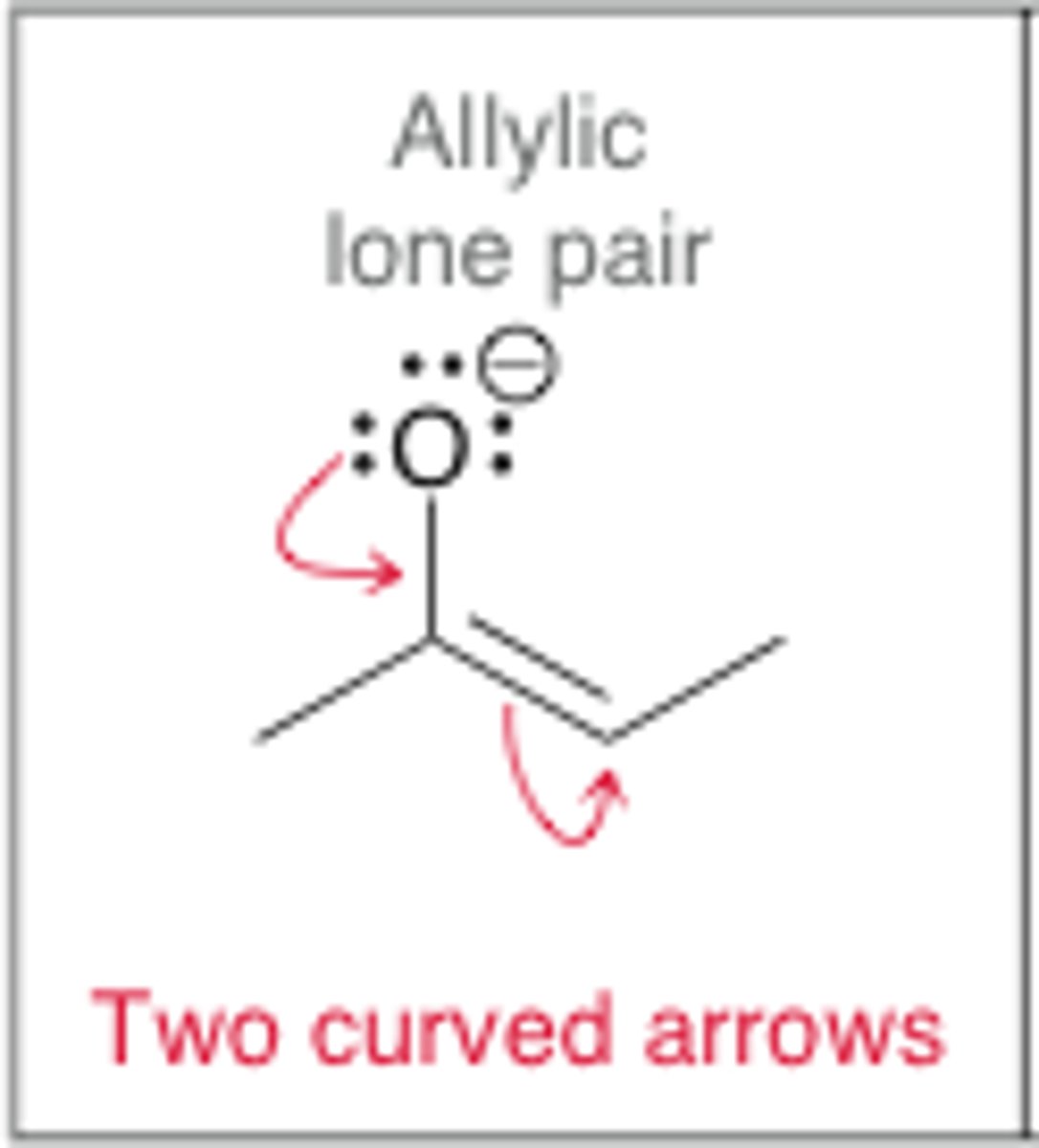
Allylic lone pair
lone pair on an atom connected to a carbon double bond
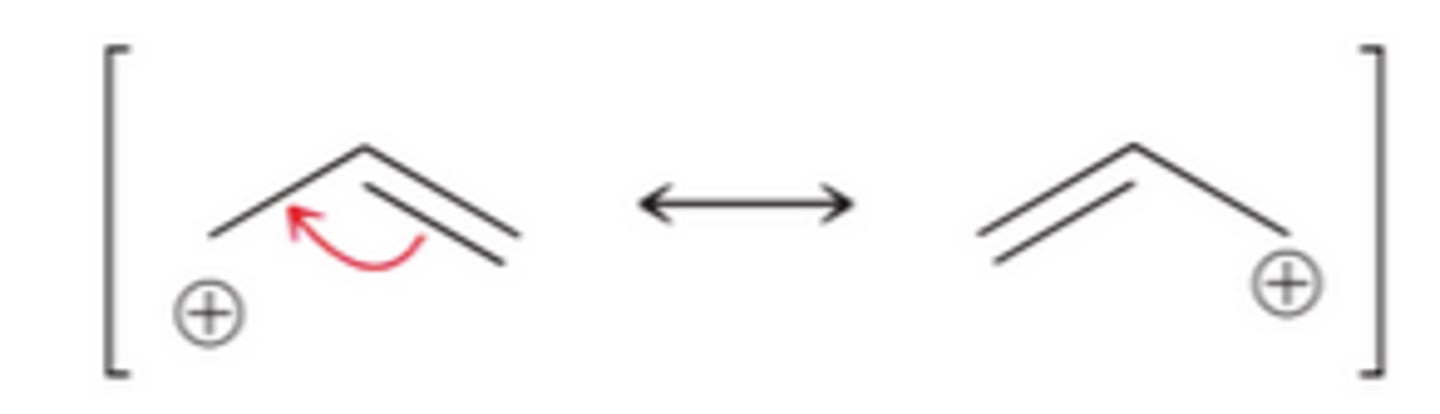
Allylic carbocation
positive charge on an atom connected to a carbon double bond
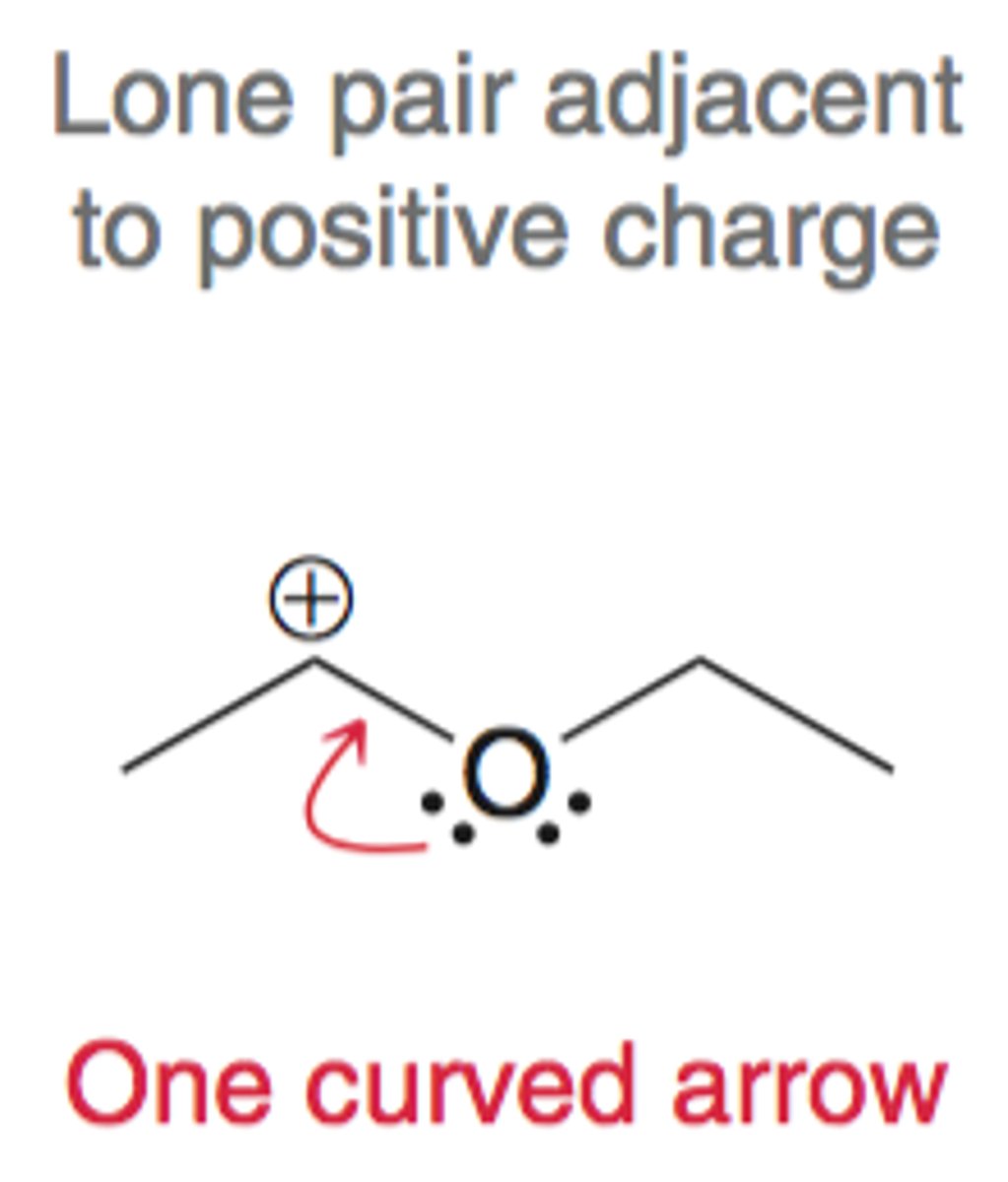
Lone pair adjacent to C+
lone pair adjacent to single carbon bond with a positive charge
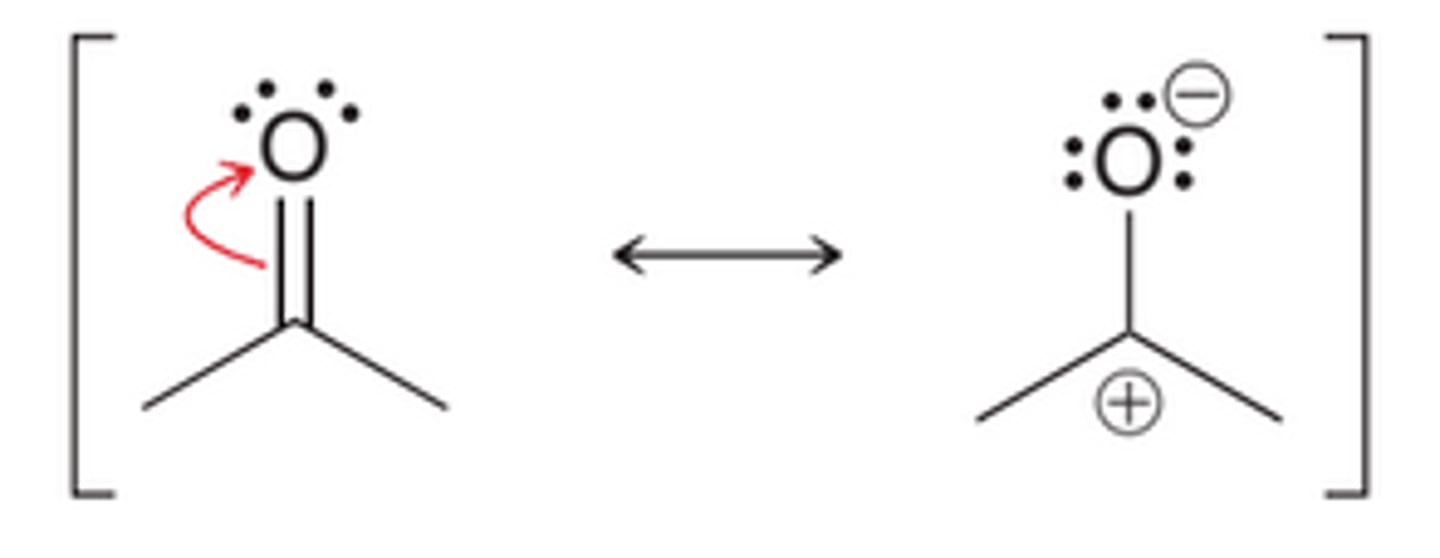
Pi bonds between 2 atoms of different electronegativity
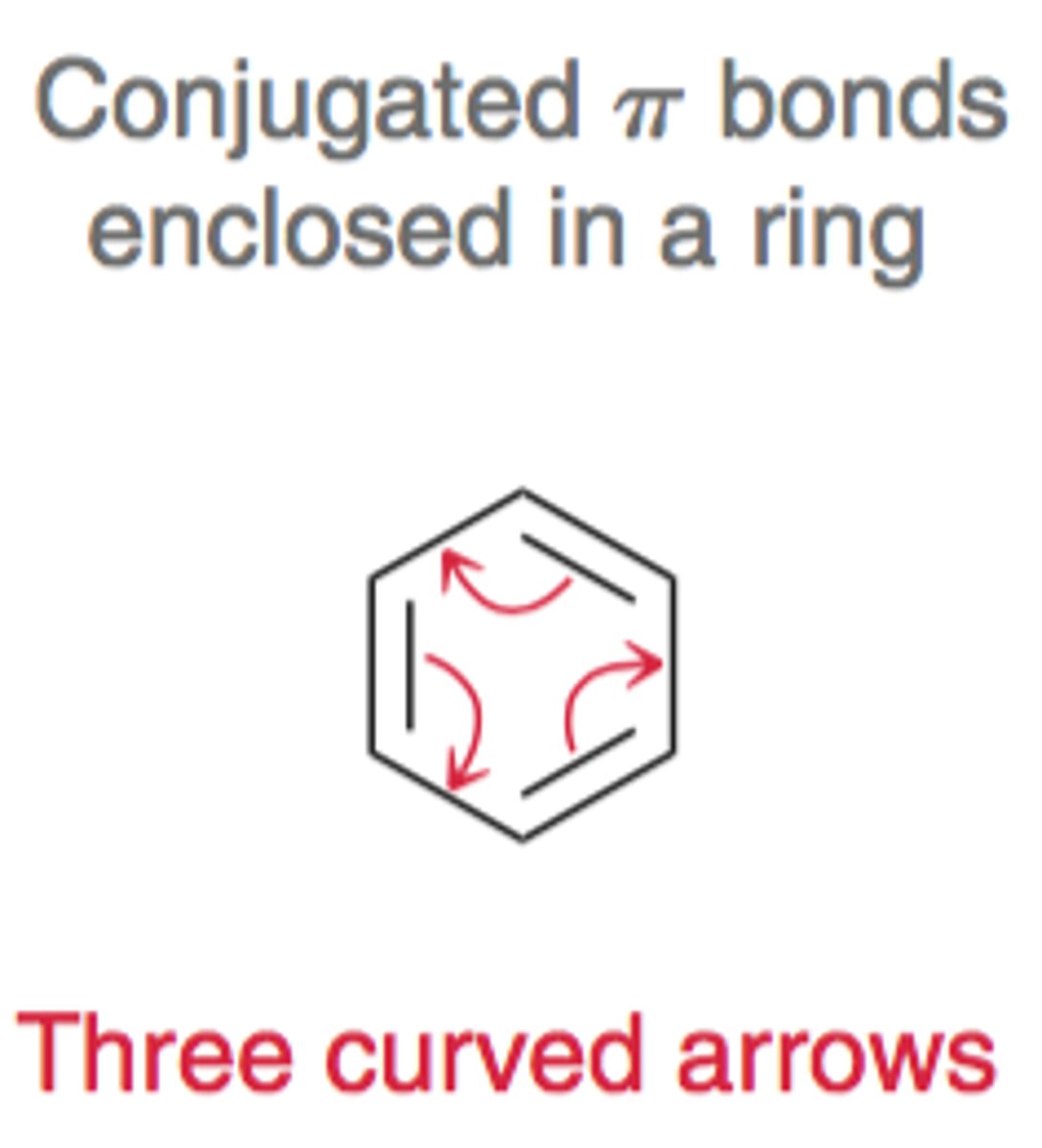
Conjugated pi bonds enclosed in a ring
List all 5 resonance structure patterns
1. Allylic lone pair
2. Allylic carbocation
3. Lone pair adjacent to C+
4. Pi bond between two atoms of different electronegativity
5. Conjugated pi bonds enclosed in a ring
3 rules of resonance structures
1. structures with all atoms having complete octet are most important
2. maximize covalent bonds
3. negative charge is most stable on most electronegative element
4. minimize charge separation
In a ring, pi bonds cannot be...
...adjacent to each other.