periodicity
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
what is periodicity? (1)
repeating trends of physical or chemical properties
what are periods in the periodic table? (1)
horizontal rows of elements in the periodic table
what are groups in the periodic table? (2)
vertical columns in the periodic table
where all the elements have the same number of electrons in their outermost principal energy level (PEL) and similar properties
where are the blocks of elements found? (4)
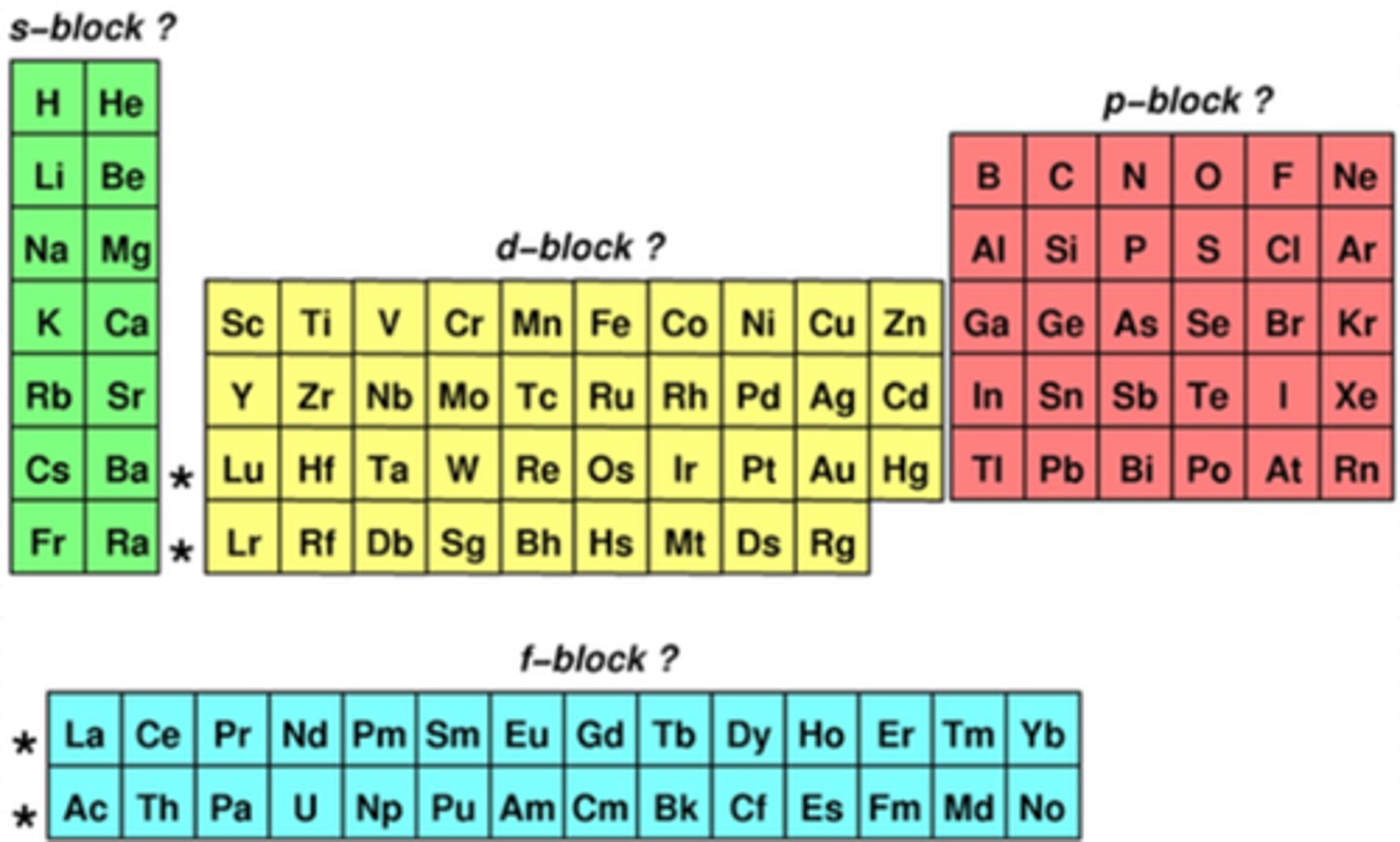
how does reactivity change in the s-block elements as we move down a group? (1)
elements get more reactive as we move down a group
how does reactivity change in non-metals as we move up a group? (1)
elements tend to get more reactive as we move up a group
how reactive are the transition metals in the d-block? (1)
usually unreactive
what type of structures do elements in groups 1, 2, and 3 have? (1)
elements in groups 1, 2, and 3 are all metals and have giant metallic structures
what type of structure does silicon (Si) in group 4 form? (1)
a macromolecular structure with 4 covalent bonds
what type of structures do elements in groups 5, 6, and 7 form? (1)
they are non-metals so form simple molecular structures
what is the structure of argon in Group 0, and why is it inert? (2)
has a simple molecular structure
with a full outer PEL of electrons (making it inert)
why do molecular structures have low melting and boiling points? (1)
they have weak Van der Waals forces between molecules that need to be broken
why do metallic structures have high melting and boiling points? (1)
due to strong electrostatic attraction between positive ions and delocalised electrons
why do macromolecular structures like silicon have very high melting and boiling points? (2)
due to strong covalent bonds which require a lot of energy to break
what type of bonding do Na, Mg, and Al possess? (1)
metallic bonding
how does the charge on the metal ion change across Na, Mg, and Al? (2)
the charge on the metal ion increases from 1+ to 3+
what happens to the number of delocalised electrons across Na, Mg, and Al? (1)
the number of delocalised electrons increases
how does the strength of metallic bonding change across Na, Mg, and Al? (2)
the strength of metallic bonding increases, making the metals harder to melt
why do Na, Mg, and Al have increasing melting and boiling points? (2)
Na, Mg, and Al have increasing melting and boiling points due to stronger metallic bonding
what type of structure does silicon (Si) have? (1)
macromolecular structure (similar to diamond)
how are silicon atoms bonded in its macromolecular structure? (2)
each silicon atom is bonded to 4 others in a tetrahedral structure
forming a giant 3D structure
why does silicon have a very high melting and boiling point? (2)
silicon has a very high melting and boiling point due to strong covalent bonds
which require a lot of energy to break
what types of structures do P, S, Cl, and Ar form? (1)
they form simple molecular structures
how do P, S, and Cl exist in nature compared to Ar? (2)
P, S, and Cl exist as simple molecules, while Ar exists as separate atoms
why do P, S, Cl, and Ar have low melting and boiling points? (2)
they have weak Van der Waals forces between molecules
requiring less energy to break and melt/boil the compounds
why does sulfur have a higher melting point than phosphorus, chlorine, or argon? (3)
phosphorus exists as P4 molecules, sulfur as S8 molecules, chlorine as Cl2 molecules, and argon as Ar atoms
sulfur, being a larger molecule, has more Van der Waals forces between molecules
more energy is required to break these forces, resulting in a higher melting point
what is the order of melting and boiling points for P, S, Cl, and Ar? (1)
S8 > P4 > Cl2 > Ar
what happens to ionisation energy down a group, and why? (5)
ionisation energy decreases
the electron is removed from a higher principal energy level
the electron is further from the nucleus
there is more shielding
weaker attraction between the nucleus and the outer electron means less energy is required to remove it
what happens to ionisation energy across a period, and why? (4)
ionisation energy increases
the number of protons increases
shielding is constant, and atomic radius decreases
stronger attraction between the nucleus and the outer electron means more energy is required to remove it
why is there an exception for Group 3 ionisation energy across a period? (3)
ionisation energy is lower
the electron is removed from a higher energy p sub-level
less energy is required to remove the electron
why is there an exception for Group 6 ionisation energy across a period? (3)
ionisation energy is lower
there is a pair of electrons in a p orbital
extra repulsion means less energy is required to remove the electron