Section 5 13 ONLY NUTRIENT CYCLES EOYS
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
Why is the nutrient cycles (phosporus and nitrogen cycle) important?
There are limited amount of nutrients in the ecosystem so recyling of these nutrients is important.
The phosphorus cycle: TO GAIN FULL MARKS GIVE AN EXAMPLE OF AT LEAST ONE BIOLOGICAL MOLECULE THAT CONTAINS PHOSPHOROUS
The phosphate ions are aborbed by the plant to make phosphate containing molecules such as ATP and DNA, they do this by mycorrhizae (mutalism the relationship between the plant and fungi) which enables the plant to absorb the phosphorous and water molecules.
Animals consume the plants and make their own phosphate containing compounds
Animals waste is then broken down by saprobionts, which releases phosphates back into the soil.
The phosphate in the soil comes to the soil as a result of weathering.
Some of the phosphates move into the water, where over thousands of years this forms new rocks.
The nitrogen cycle: TO GAIN FULL MARKS GIVE AN EXAMPLE OF AT LEAST ONE BIOLOGICAL MOLECULE THAT CONTAINS NITROGEN
Nitrogen fixation
Nitrogen gas is converted into ammonia via mutualistic nitrifying bacteria and free -living nitrogen fixing bacteria. (anaerobic conditions)
Nitrification
Ammonia ions are converted into nitrite (NO2-) and then to nitrate (NO3-) via nitryfying bacteria (using oxygen as oxidation reaction).
Ammonification:
The nitrate ions are absorbed by the plants which is incoporated in amino acids and DNA, consumed by the animals. They excrete and saprobionts bacteria convert the waste material back into ammonia ions.
Denitrification:
Where denitryfying bacteria convert some of the nitrate ions back as nitrogen gas in air. (anaerobic conditions)
The way to remember the order: Never forget, never accept defeat => nitrogen fixation, nitrification, ammonification and denitrfication
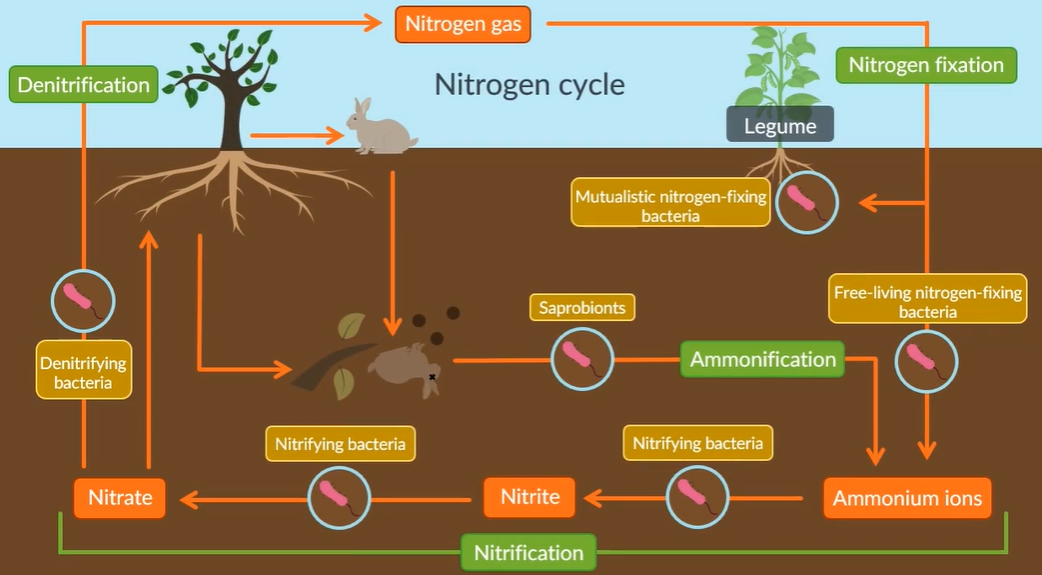
How are the nitrogen-fixing bacteria mutualistic?
The nitrogen fixing bacteria obtains carbohydrates from the plant, while the plant acquires amino acids.
The role of Mycorrhizae for plants:
They increase the surface area, increasing the rate of absporption of water and mineral ions.
Acts like a spong by holding nutrients and water in neighbouring roots.
Mutualistic relationship, the plant benefits from taking more water and mineral ions - while the mycorrhizae (fungi), that recieves sugars and amino acids
Two types of fertilisers and adv + disadv:
Natural organic fertilisers: dead, decaying remains of plant and animal matter
recycle waste, slow release of nutrients, low risk of leaching and eutrophication, bulky + smelly
Artificial inorganic fertilisers: mined from rocks, blended together to give conrrect ratio of nitrogen, phosphorus and potassium
Condensed form, highly soluble (quick release of nutrients), designed for correct balance, environmental impact for quarry + easily leached.
Effects of nitrogen-containing compounds
Reduces diversity: Only allows species that can thrive in high nitrogen soil concentrations
Causes Eutrophication: where the nitrate ions enter into water (leaching) which increases the nitrogen concentration in water which causes rapid growth of algea (algal bloom), blocks sunlight for aquatic plants and results in algea not undergoing photosynthesis, so aquatic life dies.
Sapbrobionts increase in number, which decreases the dissolved oxygen.
This results other aerobic organisms such as fish to die
Define Leaching
Process where nutrients are removed from the soil, such as nitrates and travels to the water in streams.
Define Eutraphication
When levels of nitrogen increases in bodies of water.