Molecular Geometry
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
bent, 118
2 Bonding Domains and 1 Lone Pair
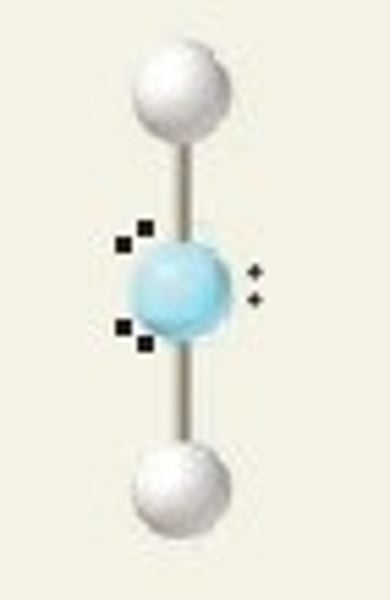
linear, 180
2 Bonding Domains and 3 Lone Pairs
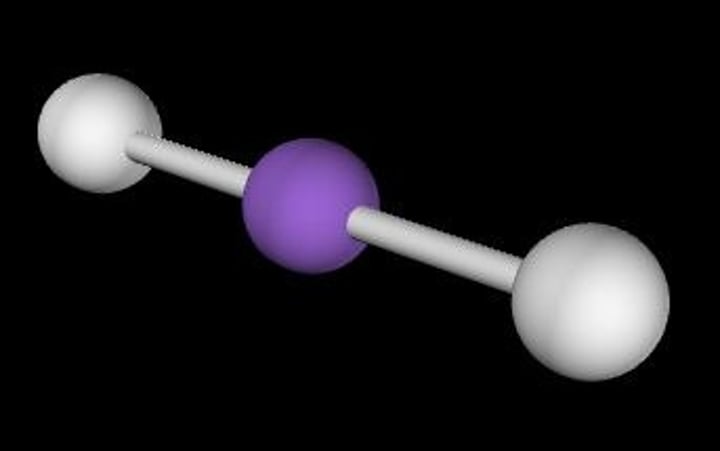
linear, 180
2 Bonding Domains and 0 Lone Pairs
octahedral, 90
6 Bonding Domains and 0 Lone Pairs
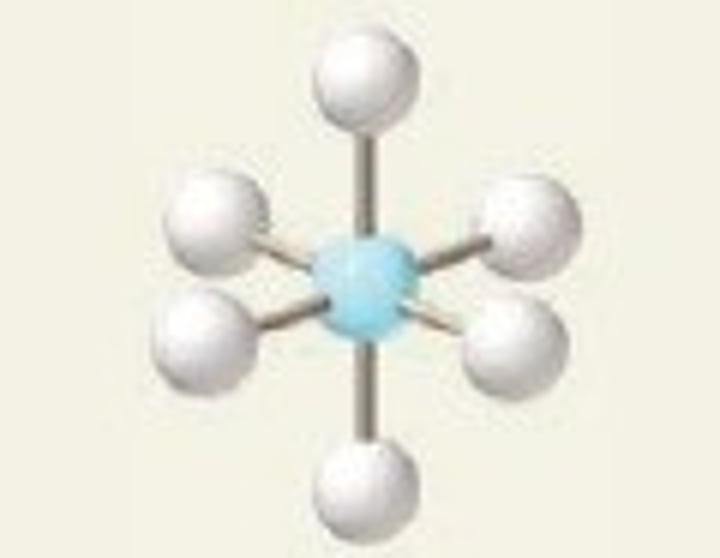
seesaw
4 Bonding Domains and 1 Lone Pair
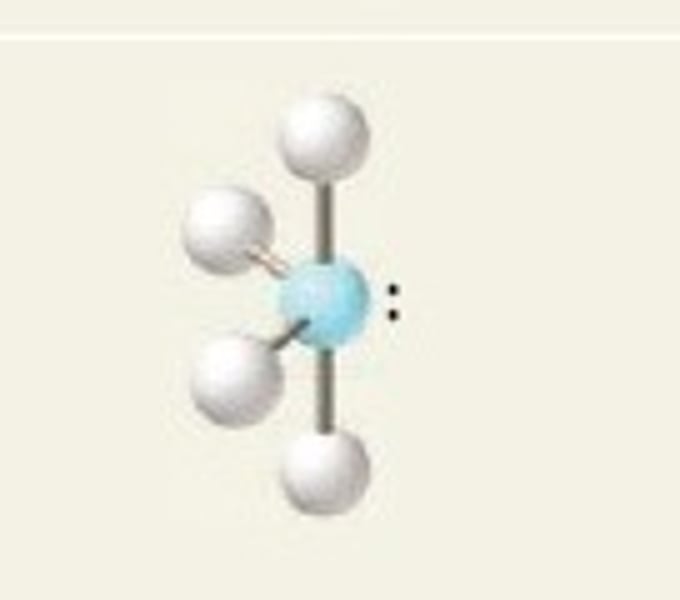
square planar
4 Bonding Domains and 2 Lone Pairs
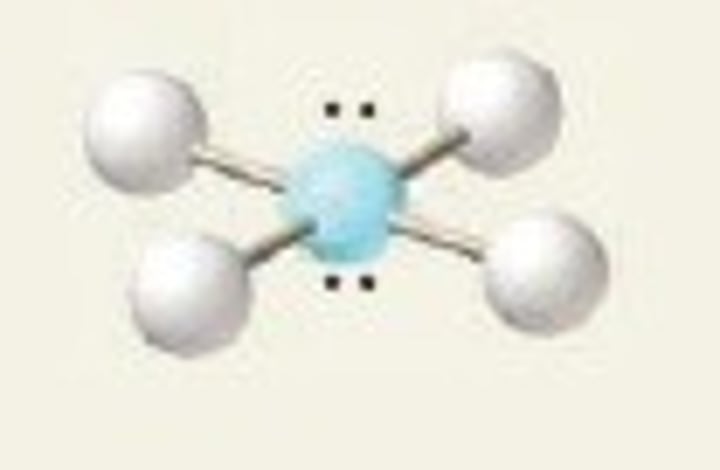
square pyramidal
5 Bonding Domains and 1 Lone Pair
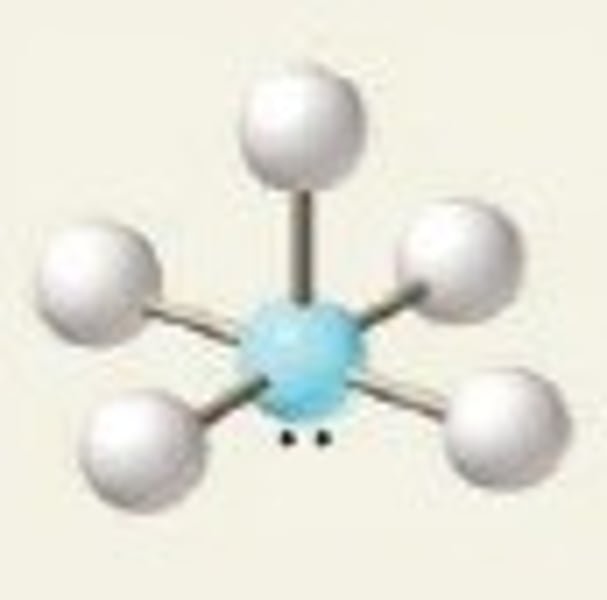
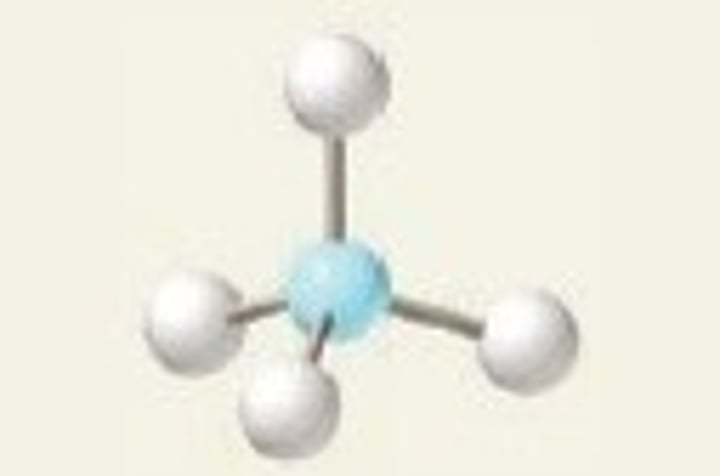
tetrahedral, 109.5
4 Bonding Domains and 0 Lone Pairs
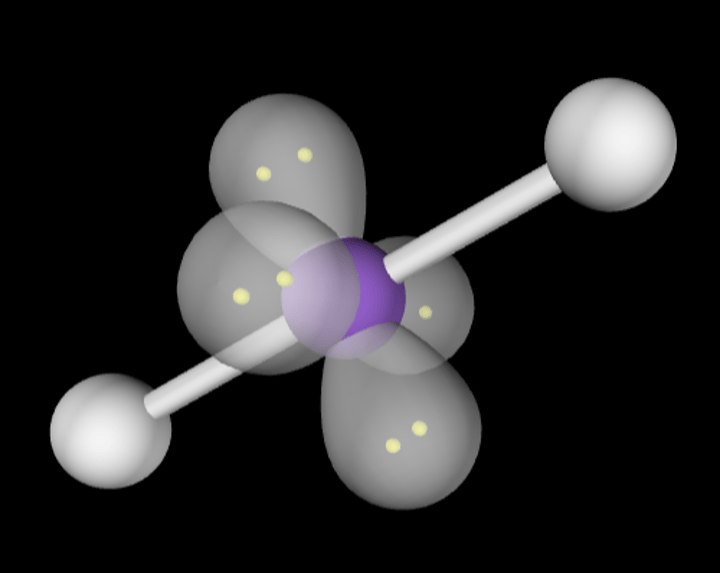
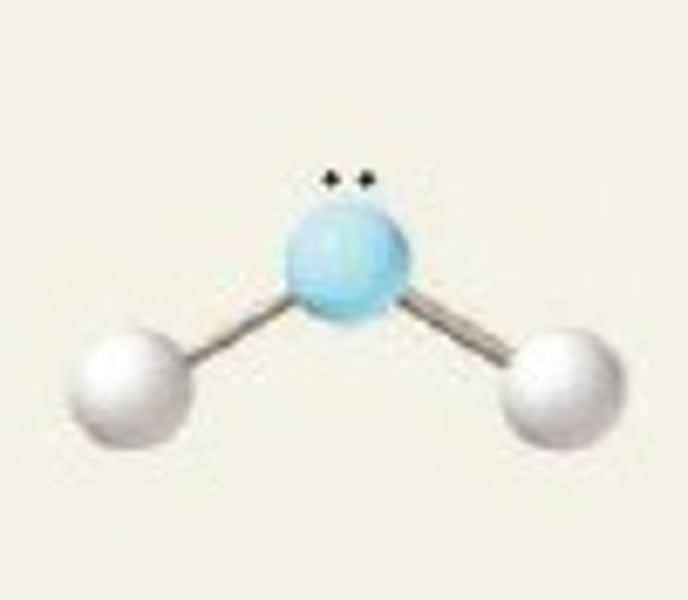
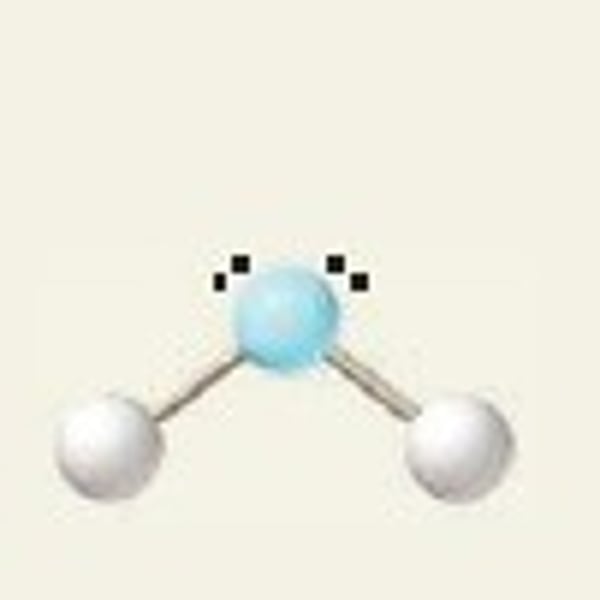
bent, 104.5
2 Bonding Domains and 2 Lone Pairs
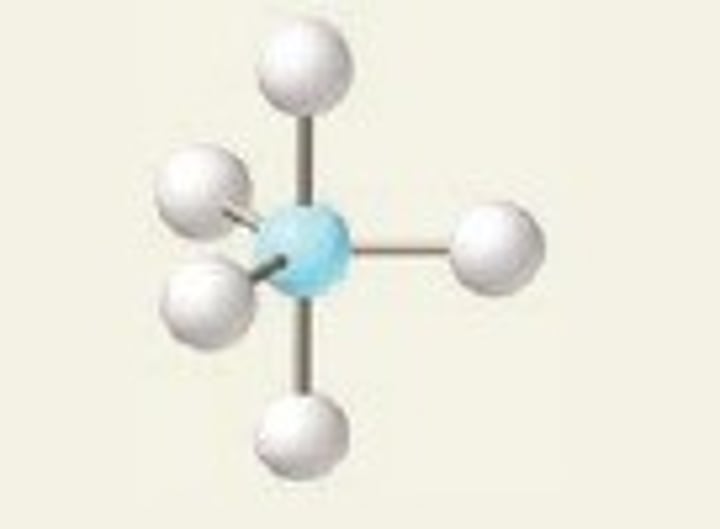
trigonal bipyramidal
5 Bonding Domains and 0 Lone Pairs
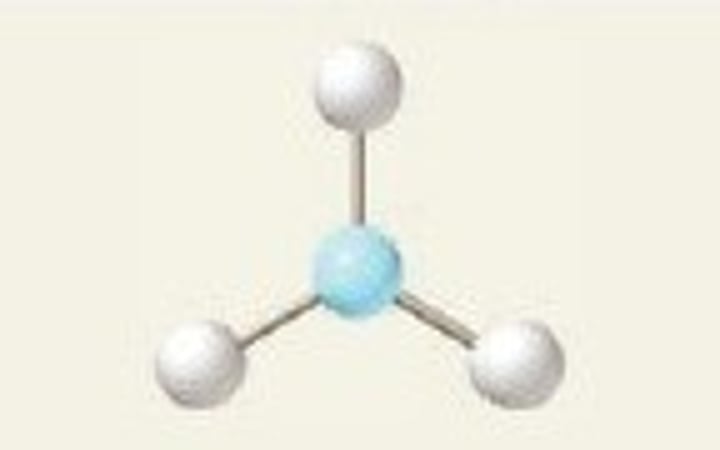
trigonal planar, 120
3 Bonding Domains and 0 Lone Pairs
trigonal pyramidal, 107.5
3 Bonding Domains and 1 Lone Pair

t-shaped
3 Bonding Domains and 2 Lone Pairs
VESPR
This theory states that pairs of electrons spread out to minimize repulsions. This theory is used to predict molecular geometry.
Shared Pair
2 electrons used to form a bond between atoms
Lone Pair
2 electrons on an atom that are not being shared; takes up more space than a shared pair
Single Bond
A bond made of 1 shared pair
Double Bond
A bond made of 2 shared pairs
Triple Bond
A bond made of 3 shared pairs
Octet Rule
Nonmetal atoms will share electrons so that they have 8 electrons in their outer shell (to have a more stable electron configuration)
Expanded Octet
atoms that can have more than an octet because they house the additional electrons in their d-orbitals; only elements in period 3 and below on periodic table can form this
t-shaped
3 Bonding Domains and 3 Lone Pairs
linear, 180
2 Bonding Domains and 4 Lone Pairs