Cell Cycle (Mitosis)
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
58 Terms
Mitotic Spindle
-Aster comes from centrosome & projects many microtubules → starts to form mitotic spindle
-Aster forms during S/G2 phase
-Mitotic spindle positions chromosomes in center during Metaphase of M-phase
microtubules are unstable
unstable because they keep growing / moving which helps them to find the center of the cell for division.
3 types of microtubules
-non -kinetochore microtubules: Motor proteins can connect them to form a gel-like structure that forms most of the spindle
-Kinetochore microtubules: span the entire length of the cell from the centrosome to the kinetochore of the chromosome
-Astral microtubules:
interphase
-Chromosomes condense
-Mitotical spindle begins to create microtubules but it is not formed yet
prophase
-This is when mitotic spindle actually forms
-Chromosomes are condensed, duplicated, and visible.
Prometaphase
-Most important event is fragmentation of nuclear envelope.
-Spindle can attach and pull easier because nucleus is not in the way.
-Kinetochore microtubules connect to kinetochores of chromosome → begins to move chromosomes
Which end of the microtubules bind to the chromosome's kinetochore?
The plus end of the microtubules
metaphase
-Equal tension of both kinetochore microtubules on either side of the chromosome pulls it into the center
-Chromosomes must be attached & lined up correctly.
Anaphase
-cohesions cleaved & leave the chromosome → sister chromatids no longer held together tightly.
-Kinetochore microtubules begin to shorten → pulls sister chromatids apart.
-Active APC/C inhibits M-cdk.
-Spindle Assembly Checkpoint to make sure chromatids properly separated
Anaphase A
chromosomes are pulled toward the poles
Anaphase B
poles of cell are pulled apart to begin 2 daughter cells
dynein
myosin
How chromatids are pulled apart
-Motor protein on end of microtubule
-Microtubule dissociates as tubulin subunits → shrinks the length → shortens to pull the chromatids apart
telophase
-When nuclear pore proteins & lamins are phosphorylated, they are disorganized.
-Nuclear pore proteins and lamins get dephosphorylated → nuclear envelope gets reformed
-Lamins attach to inside of nuclear envelope and bring chromosomes to the inside of the nuclear envelope so they don’t get shut out.
cytokinesis
-Equatorial plane of mitotic spindle gives signal to actin and myosin to start organizing under the cell membrane near the equatorial plane → creates a contractile ring of actin and myosin filaments → creates cleavage furrow.
-Daughter cells often have different amount of contents / organelles bc they have different cell fates (ex: sperm need more mitochondria, but mesophyll cells in leaves need more chloroplasts / thylakoid stacks (granum).)
What is one structure that plant cell division lacks compared to animals?
Plant cell division has no centrioles
phragmoplast microtubules
-attach to golgi vesicles
-Contains a lot of polysaccharides since it originates from golgi
-Span the entire length of cell but unlike kinetochore microtubules, they do not attach to chromosomes.
-Cell later deposits vesicles with cellulose to form cell wall between daughter cells
mitogens can control cell fate
-promote cell division
-platelet-derived growth factor
-hepatocyte growth factor
necrosis
-bad because it damages the cell & leads to inflammation
apoptosis
-Cells are "destroyed" and recycled as parts for other cells
-Caspases: enzyme that helps trigger apoptosis
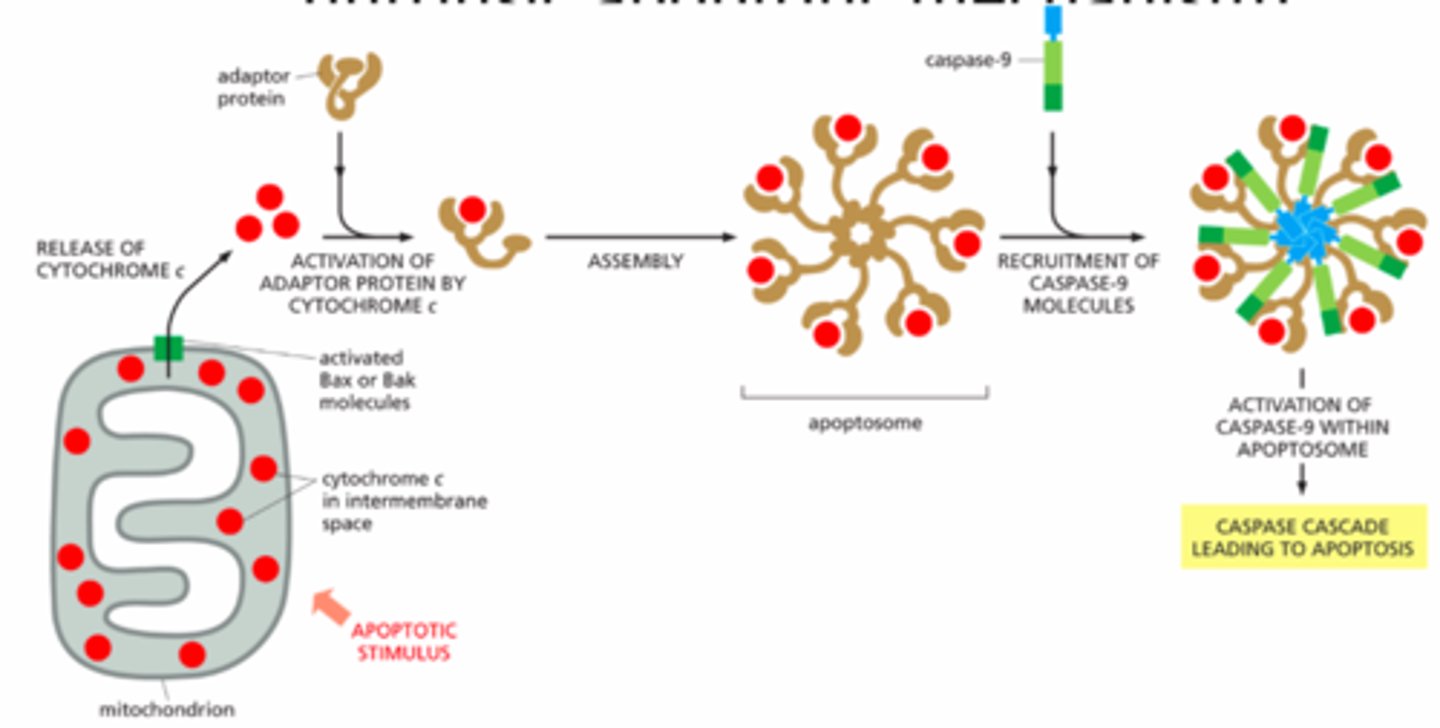
how apoptosis is regulated internally
-Mitochondria send signal to cell
-Bcl2 protein family: Some family members inhibit & some promote apoptosis.
-Bcl2 = apoptosis inhibitor
-Bax & Bak promote / trigger apoptosis
how apoptosis is regulated externally
Fas = death receptor
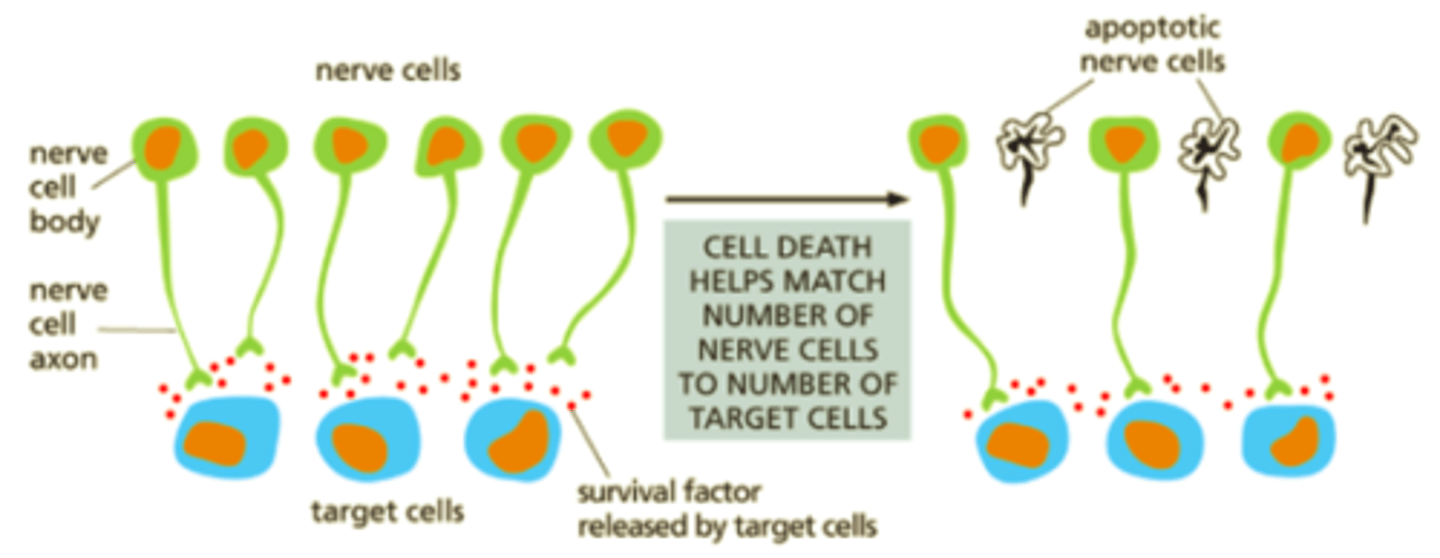
survival factors for neuronal cells
-Some Neuronal cells are made too early & too many → kill some during development.
-Target cells release survival factors → nerve cells that uptake them can block apoptosis.
-Survival factor binds to receptor → receptor activates → transcription regulator activated → transcription of Bcl2 gene → Bcl2 protein → apoptosis blocked
Cell Cycle
The ordered series of events cells go through to grow, replicate DNA, and divide; includes interphase and mitotic phases.
DNA Organization
DNA is wrapped around histones to form chromatin, which condenses into chromosomes for accurate segregation during division.
Sister Chromatids
Identical DNA copies formed during S phase; joined at the centromere and separated during mitosis.
Chromatin Condensation
A process where loosely packed chromatin becomes highly condensed into visible chromosomes during early mitosis.
Cytoskeleton in Mitosis
Supports chromosome movement and cell shape; microtubules form the spindle apparatus.
Mitotic Spindle
A dynamic microtubule structure that aligns and separates chromosomes during mitosis, organized by centrosomes.
Types of Microtubules
Includes kinetochore microtubules (attach to chromosomes), interpolar microtubules (stabilize the spindle), and astral microtubules (anchor to the cell cortex).
Interpolar Microtubules
Extend from opposite spindle poles and overlap at the cell center, helping push poles apart and stabilize the spindle.
Interphase
The cell grows, duplicates DNA, and prepares for mitosis; includes G1, S, and G2 phases.
Chromosome Condensation
Begins in late interphase; essential for ensuring chromosomes can be accurately moved during mitosis.
Prophase
Chromosomes become visible, centrosomes move to opposite poles, and the mitotic spindle begins to form.
Prometaphase
The nuclear envelope breaks down, allowing spindle fibers to attach to kinetochores on chromosomes.
Nuclear Envelope Breakdown
Triggered by phosphorylation of nuclear pore proteins and lamins during prometaphase.
Metaphase
Chromosomes align at the metaphase plate, positioned between the two spindle poles.
Metaphase Plate
An imaginary central plane where chromosomes line up before being separated into daughter cells.
Anaphase
Sister chromatids separate and move to opposite poles; driven by spindle shortening and motor proteins.
Spindle Assembly Checkpoint
Ensures each chromosome is properly attached to spindle fibers before anaphase begins; prevents chromosome missegregation.
Kinetochore Signal
If a kinetochore isn't properly attached, it sends a "stop" signal to delay progression into anaphase.
Anaphase A and B
A: Chromatids move toward spindle poles. B: Spindle poles push apart to stretch the cell.
Telophase
Chromosomes decondense and nuclear envelopes reform; marks the end of nuclear division.
Nuclear Reassembly
Involves dephosphorylation of nuclear proteins and begins in late anaphase, restoring the nucleus.
Cytokinesis
Cytoplasmic division that follows mitosis; results in two separate daughter cells.
Cleavage Furrow
A contractile ring that pinches the plasma membrane to physically divide the cell during cytokinesis.
Contractile Cortex
A layer of actin and myosin beneath the cell membrane that drives the formation of the cleavage furrow.
Plant Cell Division
Lacks centrioles; uses vesicles to form a cell plate that becomes the new cell wall.
Vesicle-Mediated Cell Plate
Vesicles deliver polysaccharides and glycoproteins to the center of the plant cell; cellulose is added to complete the new wall.
Mitogens
Signaling proteins that stimulate cells to divide, often by activating pathways that drive progression through the G1/S checkpoint.
Growth Factors
Extracellular signals that increase cell size and biosynthesis by promoting protein and lipid production.
Survival Factors
Prevent programmed cell death (apoptosis), allowing cells to persist under favorable conditions.
Apoptosis vs. Necrosis
Apoptosis is a controlled cell death process; necrosis is uncontrolled and often results from injury.
Apoptosis in Development
Helps sculpt tissues (e.g., removing webbing in fingers); also removes damaged or unnecessary cells.
Caspases
Proteases activated in apoptosis; cleave cellular components in an orderly way to dismantle the cell.
Bcl2 Family Proteins
Regulate apoptosis by controlling mitochondrial membrane permeability; some promote death (Bax, Bak), others prevent it (Bcl2).
Fas (Death Receptor)
A membrane receptor that initiates extrinsic apoptosis when bound by Fas ligand, leading to caspase activation.