The immune response in time and space
1/47
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
48 Terms
What is the immune response?
A co-ordinated response to protect the body
against trauma and infection and to maintain homeostasis and generate future protection.
How long does the immune response take?
10 - 14 days
Where does lymphocyte activation primarily take place?
In the secondary lymphoid organs
Which organs are included in the secondary lymphoid organs?
Lymph nodes, the spleen, and mucosa-associated lymphoid tissue (MALT).
Where else can lymphocyte activation occur besides secondary lymphoid organs?
At sites of inflammation, also known as tertiary lymphoid organs.
What causes secondary or tertiary lymphoid tissues to swell?
Proliferation of lymphocytes in response to antigen
What is a common example of swelling due to lymphocyte proliferation?
Swollen glands.
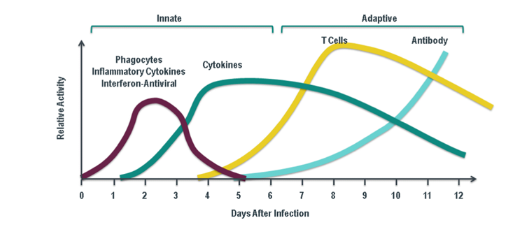
What are the sequence of events in dealing with individual pathogens?
Pathogens are dealt with in three sequential phases—first line, second line, and third line of defence (1 → 2 → 3). Although each pathogen passes through these phases in order, multiple infections can cause the phases to overlap over time.
What are the three major factors that determine an immune response?
(1) The nature of the antigen or pathogen—whether it is intracellular or extracellular and its structure
(2) whether it is a first exposure or a subsequent exposure
(3) the location and condition of the body at the time of exposure.
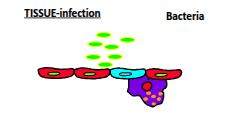
What makes up the 1st line of defence (barrier immunity)?
Epithelial cells, resident macrophages, lysozyme, and anatomical factors such as pH, mucous, commensal microbes, and physical expulsion mechanisms like coughing and sneezing.
Macrophages from different tissues…
• Liver-Kupffer cells
• Kidney-Mesangial cells
• Central nervous system-Microglia
• Connective tissues-Histiocytes
• Bone-Osteoclasts
• Lung-Alveolar macrophages
• Spleen-Littoral cells
• Joints-Synovial macrophages
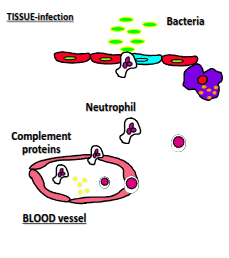
What occurs in the 2nd line of defence (innate immunity)?
PAMP activation of TLRs, directed chemokine secretion (e.g. IL-8), recruitment of neutrophils and monocytes, phagocytosis and killing of pathogens, apoptosis, resolution of inflammation, and activation of complement proteins.
What happens in TLR pathways?
TLRs recognise PAMPs and trigger intracellular signalling pathways involving key transcription factors (NFκB, IRF, and AP-1), leading to the production of cytokines, chemokines, anti-microbial peptides, and antiviral cytokines such as IFN-α and IFN-β.
What are TLRs and what is their function?
Toll-like receptors (TLRs) are pattern-recognition receptors that detect pathogen-associated molecular patterns (PAMPs) and activate immune responses, including cytokine production and inflammation.
What occurs in the 3rd line of defence (adaptive immunity)?
Dendritic cells sample antigens and present them on MHC class II, lymphocytes recirculate through the body, and T and B cells undergo clonal selection, clonal expansion, and differentiation to generate a targeted adaptive immune response.
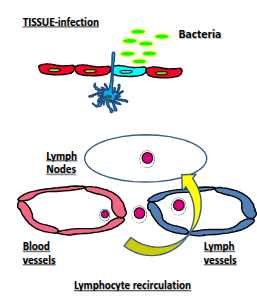
What is unique about lymphocyte recirculation compared with other leukocytes?
All leukocytes can enter inflamed tissues, but only lymphocytes continuously move between the blood and tissues even without inflammation, enabling immune surveillance.
Why is lymphocyte recirculation important?
It ensures the right lymphocyte is in the right place at the right time, as the body contains around 10¹² lymphocytes but each specificity is rare—only about 1 in 10,000 can recognise a given antigen.
Where in the body are lymphatic vessels particularly abundant?
They are rich in tissues that frequently encounter foreign antigens, such as the skin and mucous membranes. Lacteal lymphatic vessels in intestinal villi also absorb dietary lipids.
Where are lymphatic vessels less common in the body?
They are less branched in the brain, cartilage, and other avascular tissues, although major clusters exist in areas such as the groin, neck, and axillae.
What is the general flow of fluid into the lymphatics?
Plasma is filtered from the high-pressure arterial side of capillaries into the interstitial space, and excess fluid and macromolecules then enter lymphatic vessels through permeable lymphatic endothelial junctions.
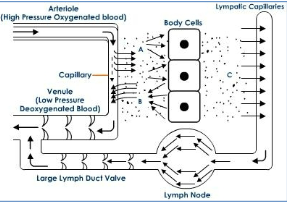
What is the key anatomical difference between blood vessels and lymphatic vessels?
Blood vessels form a closed circulatory system, whereas lymphatic vessels are blind-ended, one-way tubes that transport tissue fluid and leukocytes.
How do lymphatic capillaries differ structurally from blood capillaries?
Lymphatic capillaries are thin-walled (about 30–80 nm in diameter) and lack pericytes, which normally surround blood capillaries.
What feature of lymphatic capillaries allows immune cells to enter them?
They have a discontinuous basement membrane with portals that permit cells such as dendritic cells to migrate into the vessels.
What unique structures do lymphatic endothelial cells (LECs) have that form the primary lymphatic valves?
LECs have flap-like openings that form the primary lymphatic valves, allowing access to the vessel lumen
What role do anchoring filaments play in lymphatic endothelial cells (LECs)?
Anchoring filaments attach LECs to collagen fibres and regulate the valve-like opening into the lymphatic vessel lumen.
How does the shape of lymphatic endothelial cells (LECs) compare to blood vascular endothelial cells (BECs)?
LECs are a single layer of oak-leaf–shaped cells, which differ from the typical BECs
What type of endothelial junctions do blood endothelial cells (BECs) have?
BECs have zipper-like endothelial junctions
How do lymphatic endothelial cells (LECs) differ in their junctions compared to blood endothelial cells?
LECs have discontinuous button-like junctions, allowing fluid and certain leukocytes to pass through.
Why are the button-like junctions of LECs functionally important?
They allow fluid and specific leukocytes to enter the lymphatic vessel lumen.
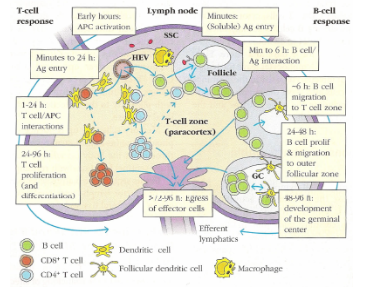
Describe the journey and activation of B cells in the lymph node after circulating with CD4 and CD8 T cells.
B cells enter follicles (B cell zones) scanning for unprocessed antigen, which can arrive within minutes and is passed to follicular dendritic cells (DCs). B cells bind antigen on follicular DCs (~6 hours), receive T cell help, proliferate (24–48 hours), form germinal centres for antibody diversification and memory cell development (48–96 hours), then are released back into the circulation.
Describe the activation and fate of T cells in the lymph node.
T cells scan for processed antigen presented on dendritic cells (DCs) in the paracortex. DCs carrying antigen arrive within hours. T-cell/DC interactions last 1–24 hours, followed by T-cell proliferation and differentiation (24–96 hours), and finally T cells are released back into the circulation after more than 72 hours
In the lymph node......
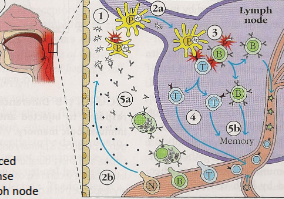
How do innate and adaptive immunity collaborate following a pathogen introduction?
1) Pathogen is introduced, triggering a phagocytic response.
2a) Pathogen or antigens are transferred to a lymph node; 2b) Some cells home to the site of infection.
3) Antigen presentation to T and B cells activates lymphocytes, which are then released into circulation.
4) Continued interactions in the lymph node drive lymphocyte proliferation and differentiation.
5a) Antibodies are produced to label pathogens; 5b) Some T and B cells persist as memory cells.
Clonal selection and generation of effector and
memory lymphocytes
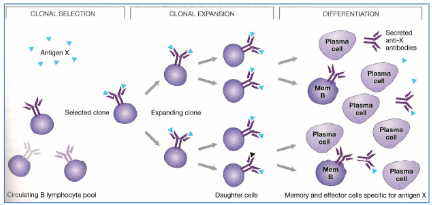
Antigen interactions in B, Tc and Th cells
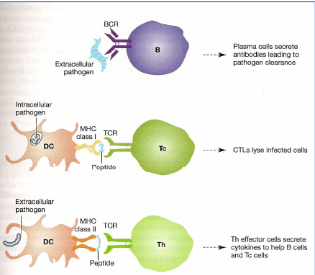
Describe the primary and secondary immune responses on repeated infection.
First exposure: specific antibodies and T cells are produced. Second exposure: early reinfection is cleared by preformed immune reactants. Third (or later) exposure: late reinfection triggers a very rapid increase in antibodies and specific T cells.
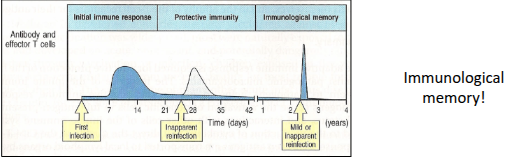
What occurs during the primary immune response regarding antibodies and T-cell help?
Initial production of IgM occurs 3 days–2 weeks after antigen exposure. T-cell help promotes class switching to IgG, IgA, and IgE after ~8 days. By 2 weeks, IgG becomes the majority antibody. After the initial lag, IgG increases logarithmically. IgG has a 23-day half-life, and long-lived plasma cells may survive for years, shaping the strength and nature of the response.
How does the secondary immune response differ from the primary response?
Secondary responses feature higher numbers of plasma cells, longer antibody half-life, and memory B cells that rapidly produce high-affinity IgG from IgM. There are higher antibody titres, faster responses, increased concentrations of T cells, and the presence of memory T cells, resulting in a stronger and quicker immune reaction
What characterises antibodies in the primary immune response?
Antibodies come from a diverse population of plasma cells recognising different epitopes, with varying affinities. They generally have low affinity and few somatic mutations
What characterises antibodies in the secondary immune response?
Antibodies are derived from a more limited population of plasma cells, recognising fewer epitopes but with very high affinity and extensive somatic mutations
How is immunological memory maintained?
Immunological memory is maintained through several mechanisms: long-lived plasma cells, antigen-independent polyclonal activation, and persistent activation by the same antigen.
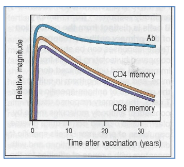
How does the secondary immune response behave after polio vaccination?
Antibodies show no significant decline, with half-lives exceeding 20 years. T cells have a half-life of 8–15 years, indicating long-lasting immunity
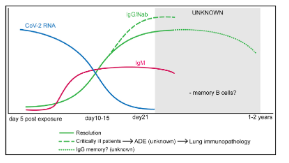
How does the secondary immune response behave after COVID infection?
Virus-specific IgM and IgG appear within 7–14 days. Antibody titres inversely correlate with viral RNA. The antibody response is short-lived, and there is no strong evidence for long-lived B cells
What happens to pathogen levels and proliferative capacity at the end of an immune response?
Pathogen levels decrease, and the proliferative capacity of immune cells declines.
How do effector cells, such as neutrophils, end the immune response?
Effector cells have limited lifespans (e.g., neutrophils ~5 days) and undergo apoptosis
What role do Fas-FasL interactions play in ending the immune response?
T cells express the death receptor Fas, and activated T cells express FasL, triggering apoptosis to remove excess T cells
How do regulatory T cells and cytokines contribute to the end of the immune response?
Regulatory T cells and anti-inflammatory cytokines help suppress immune activity and restore homeostasis
What happens to the lymph node and RNA regulation after an immune response?
The lymph node returns to a naive state, and extracellular regulatory RNAs, including microRNAs, help modulate immune activity.