BBMB 221: Exam 2
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
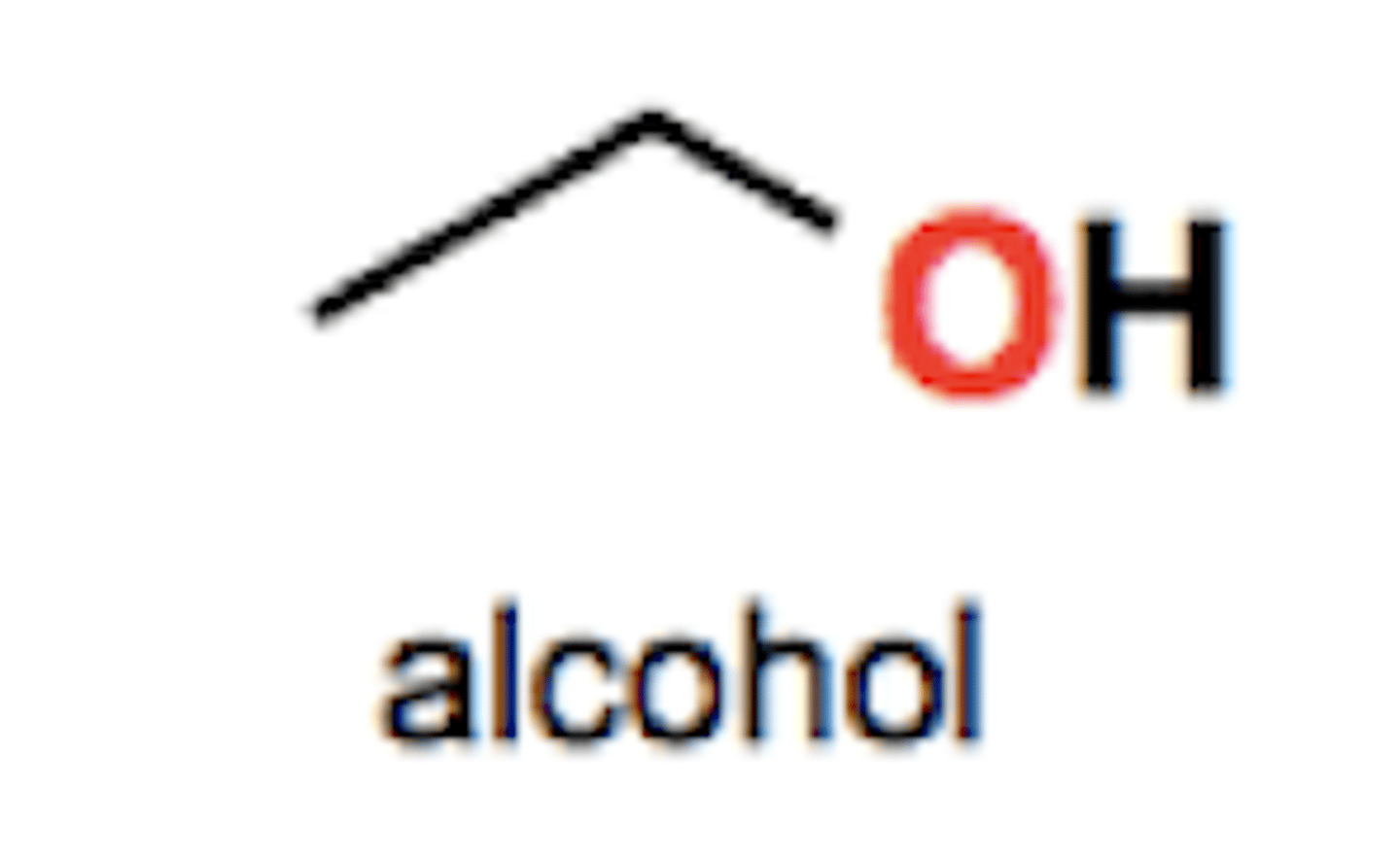
Alcohol group structure
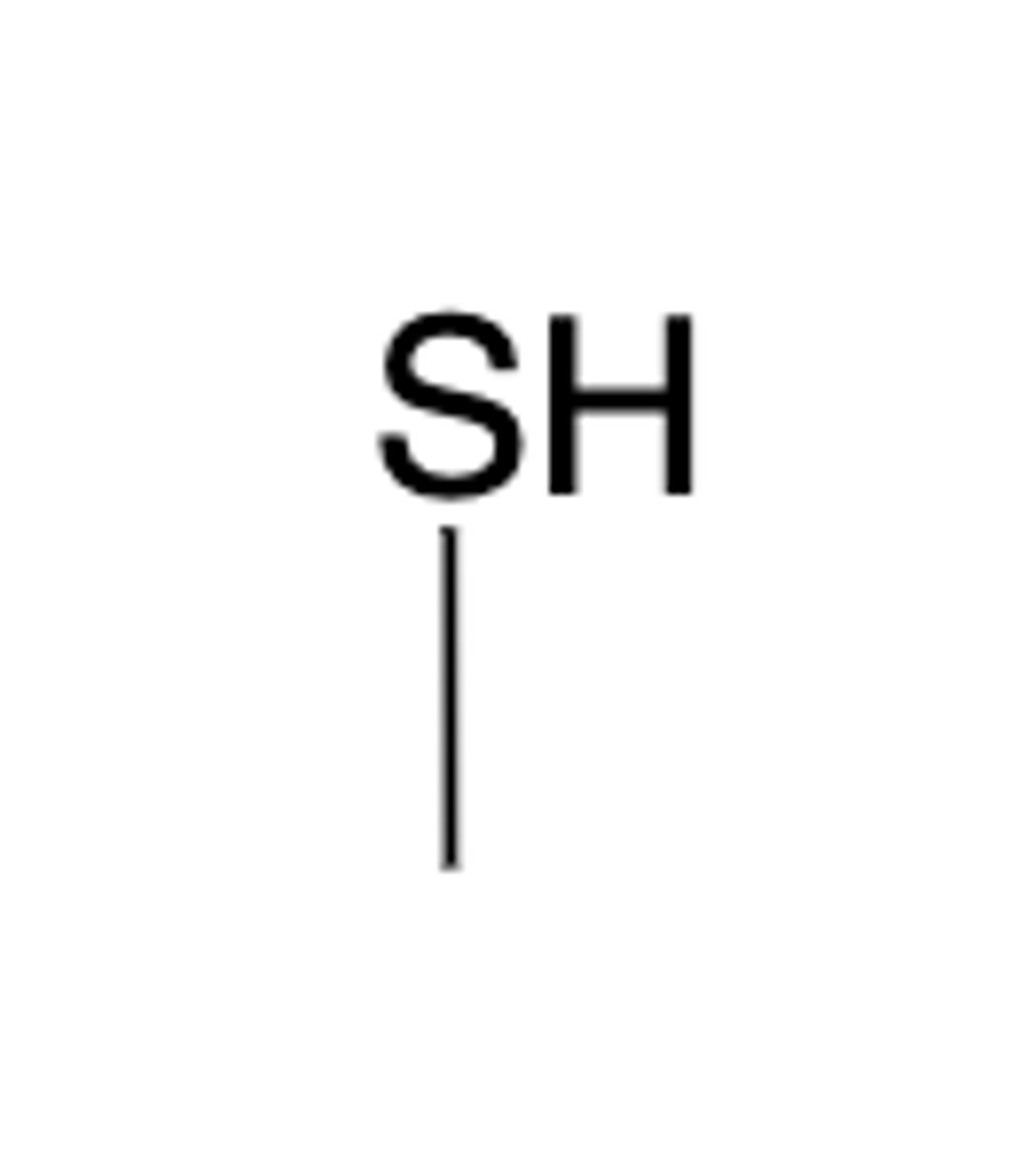
Thiol group structure
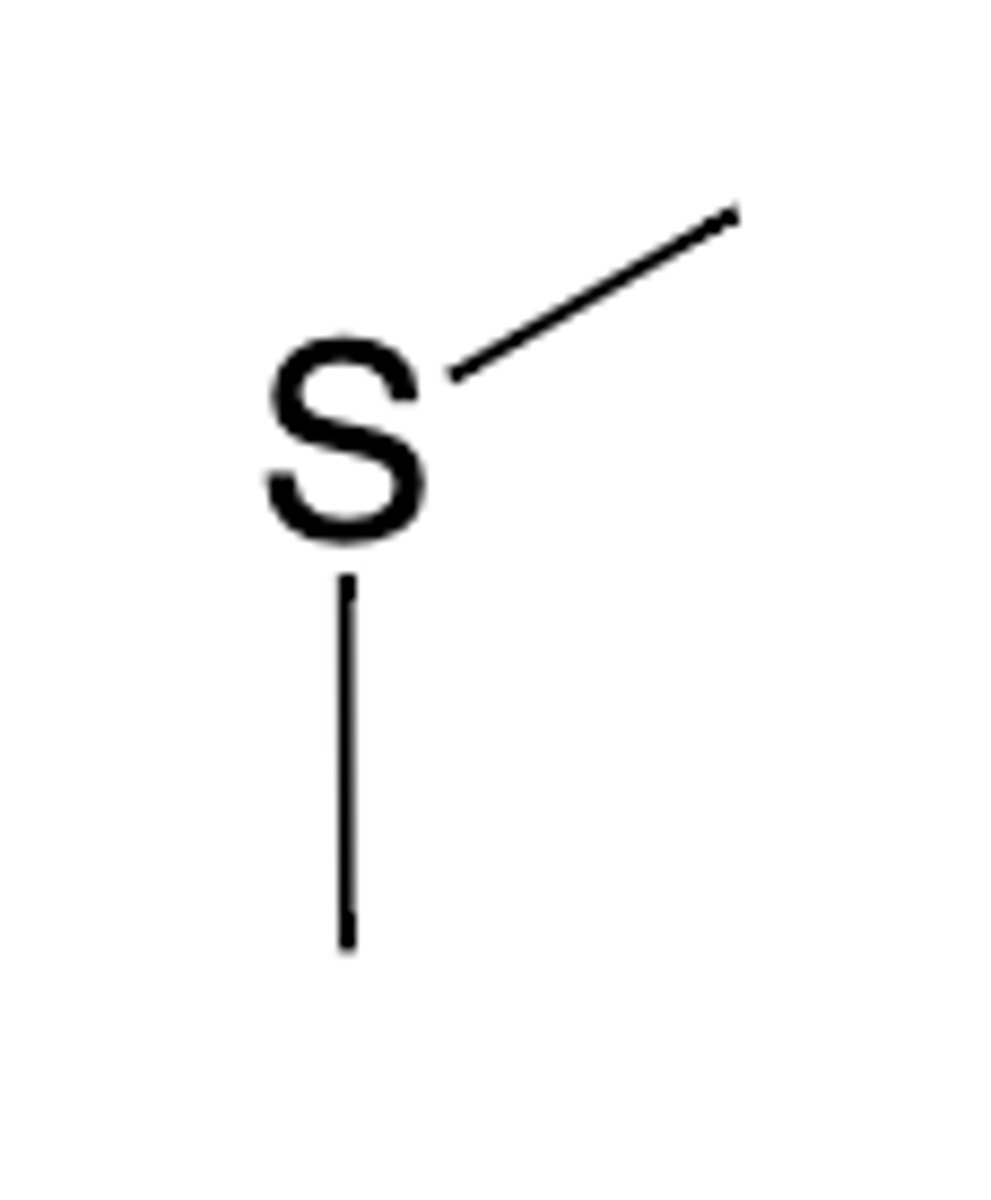
Tioether group structure
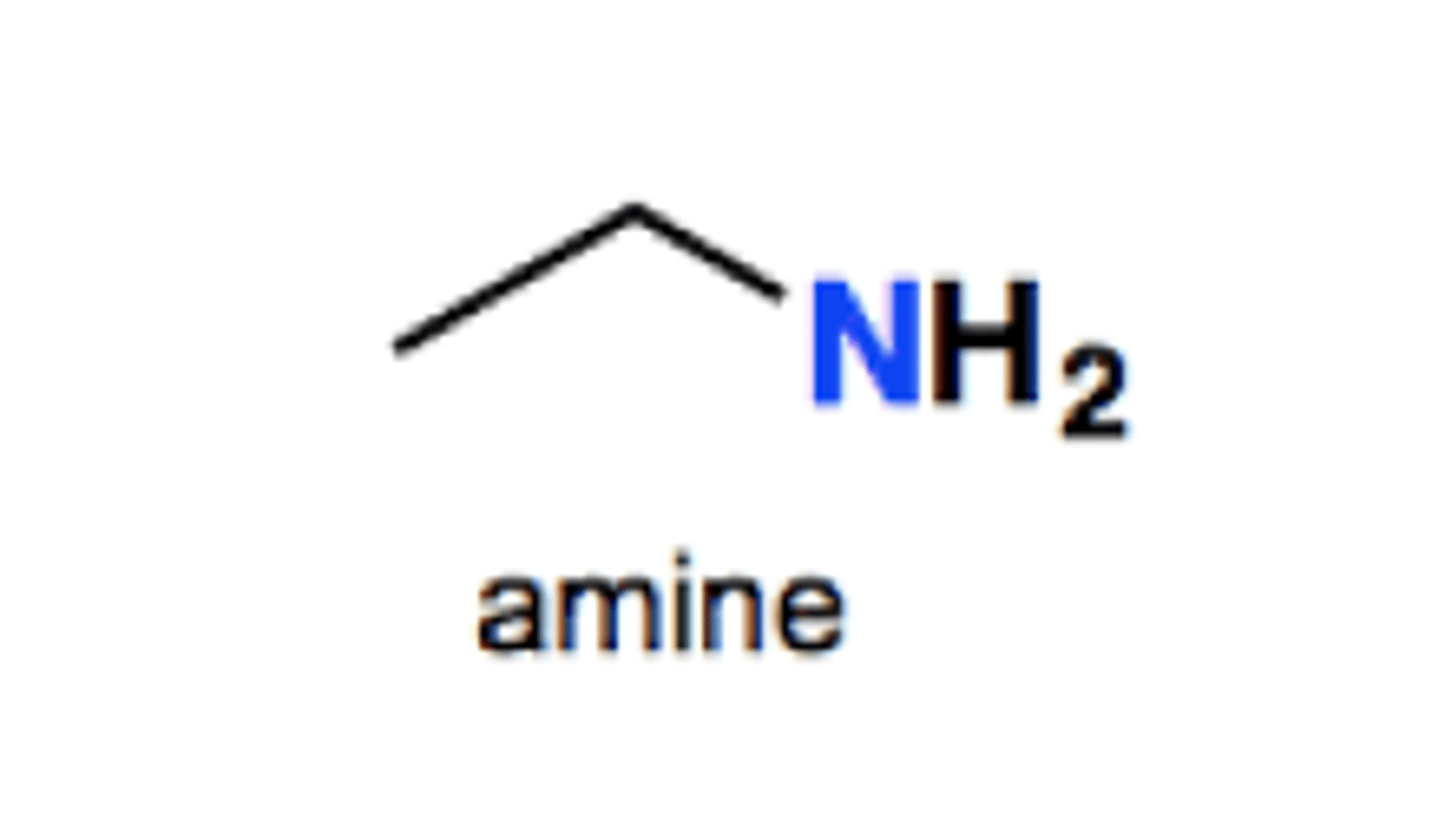
Amine group structure
Aldehyde group structure
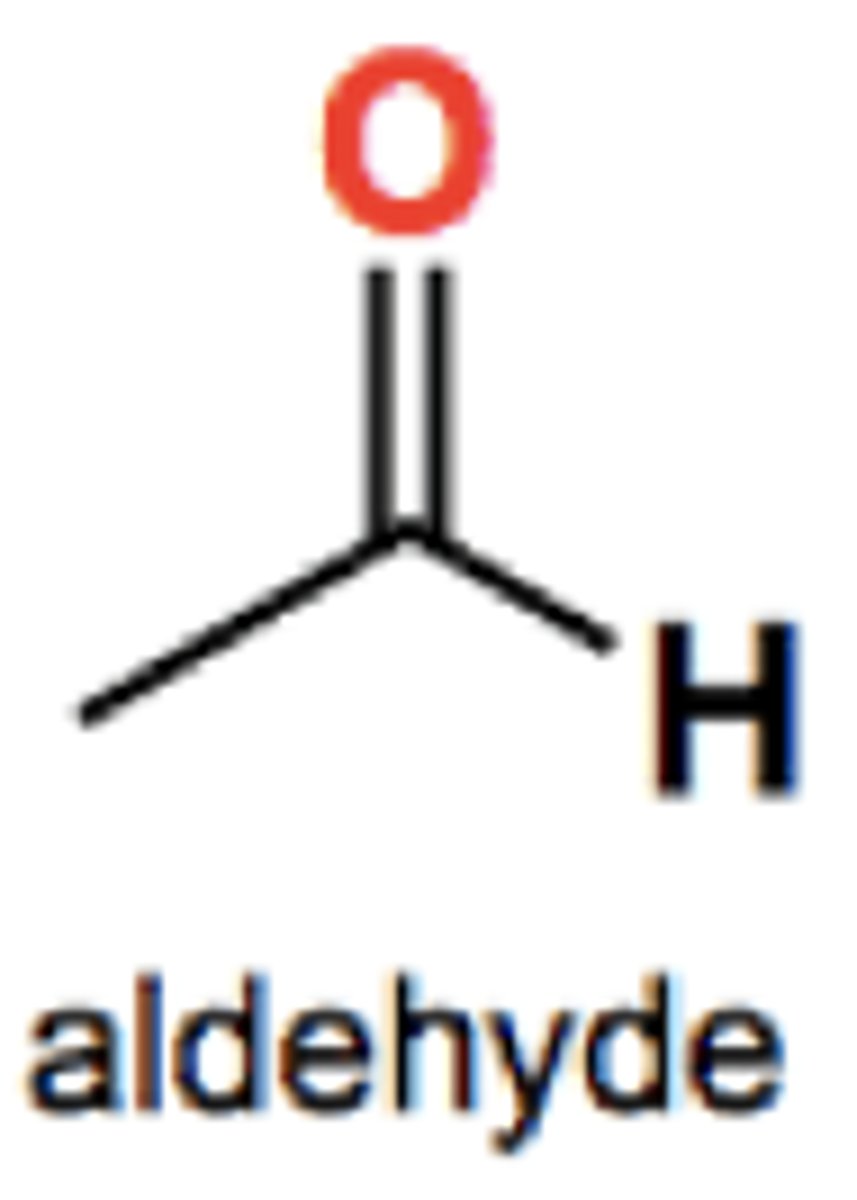
Ketones group structure
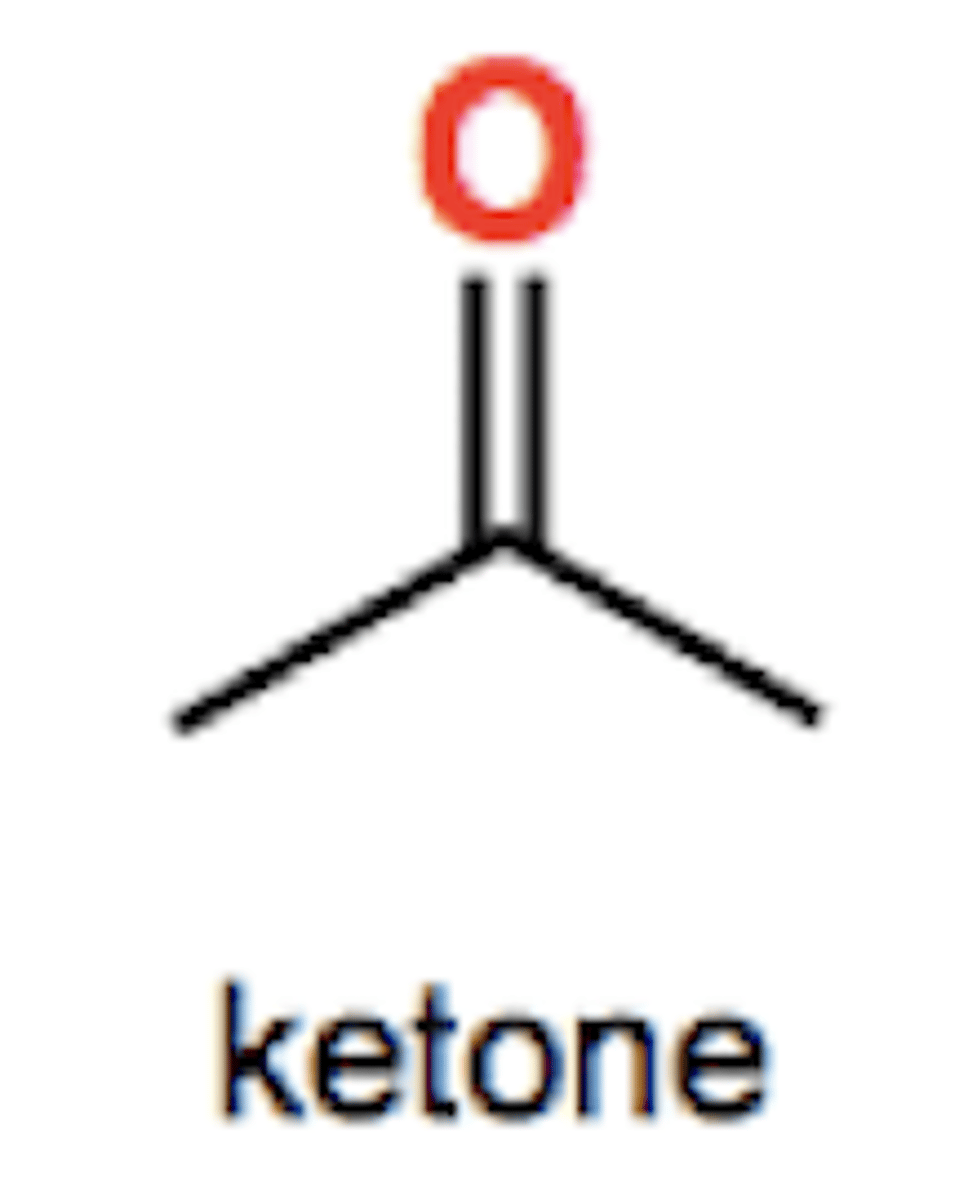
Carboxylic acid group structure
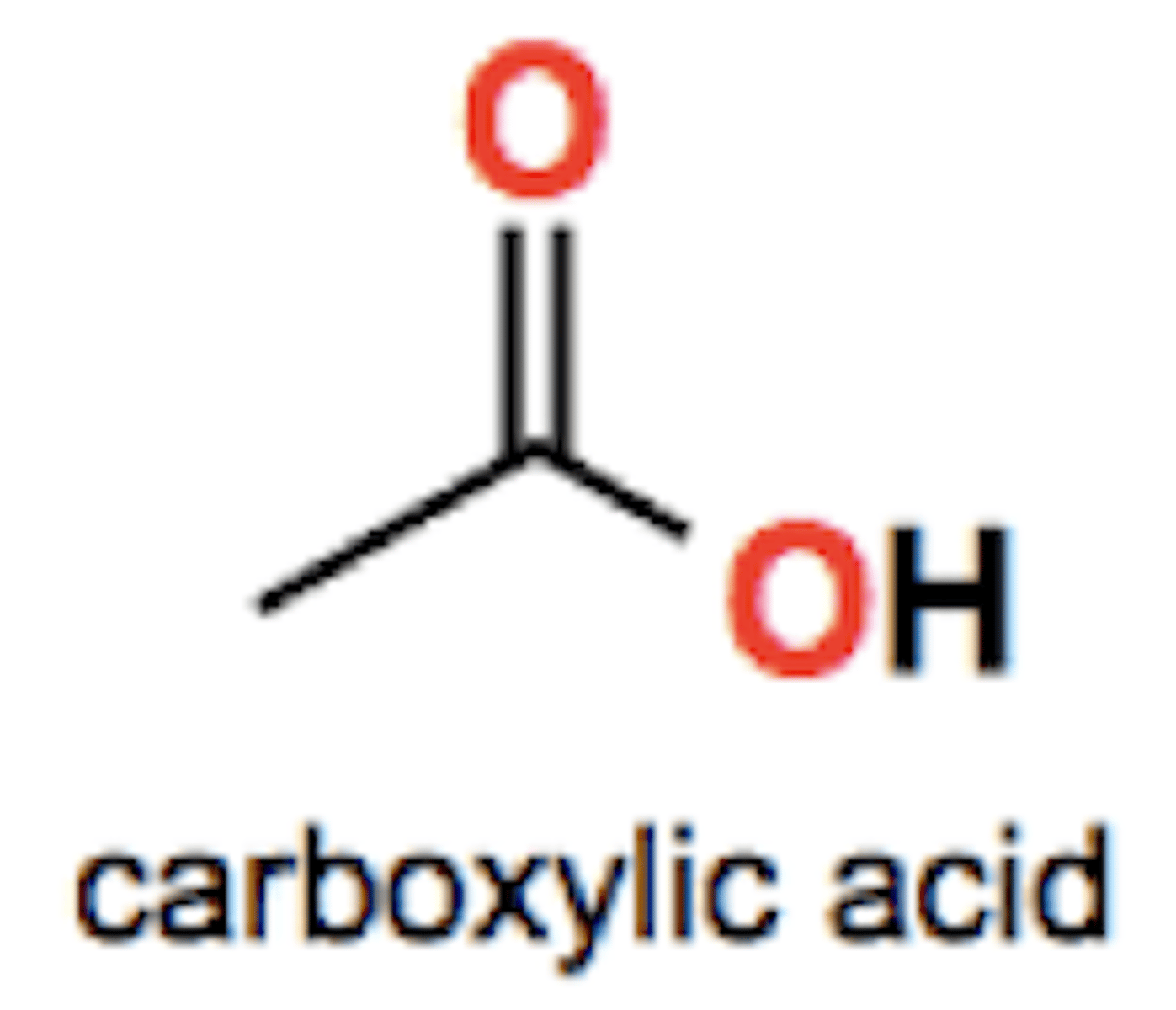
Ester group structure
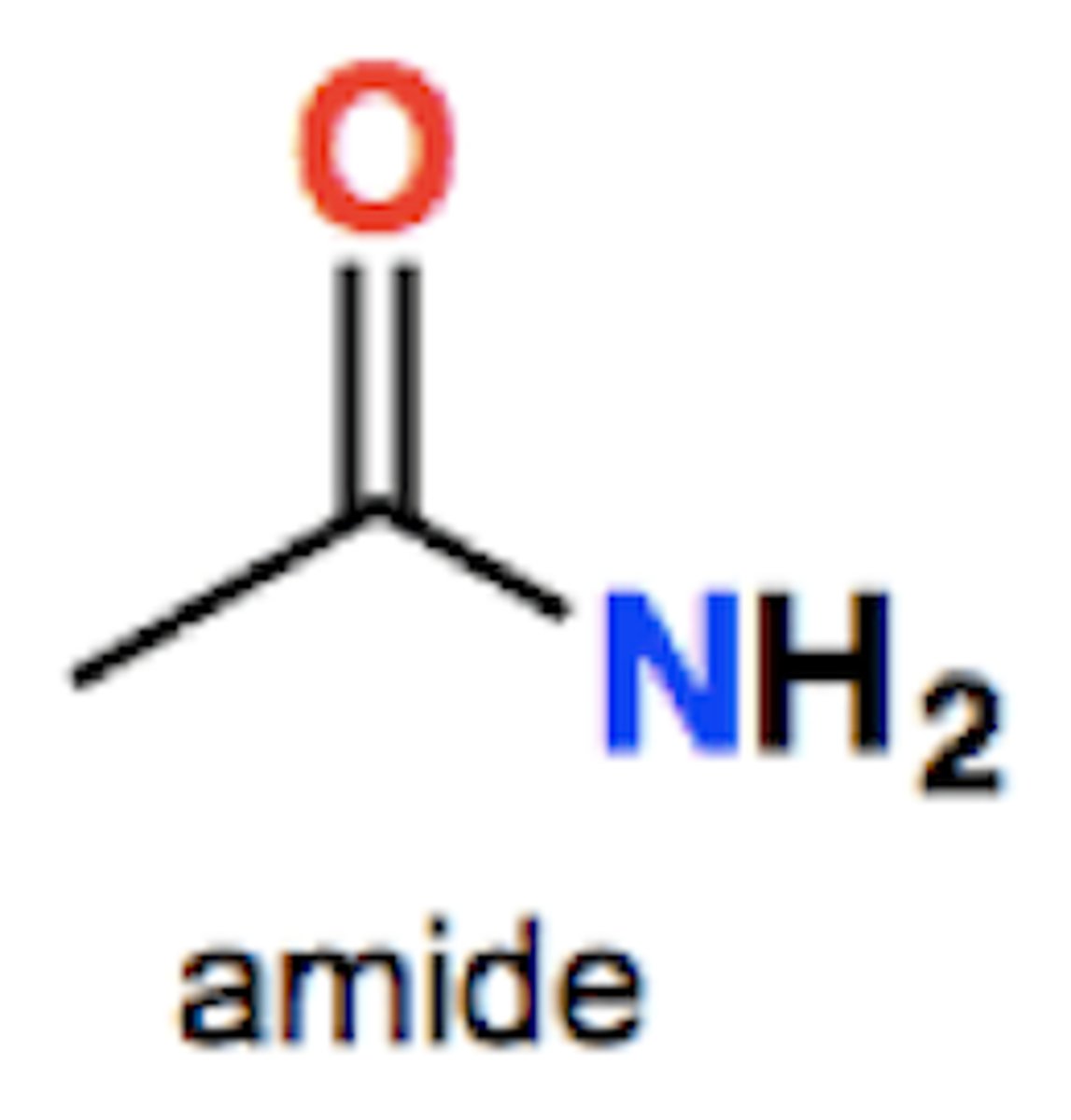
Amide group structure
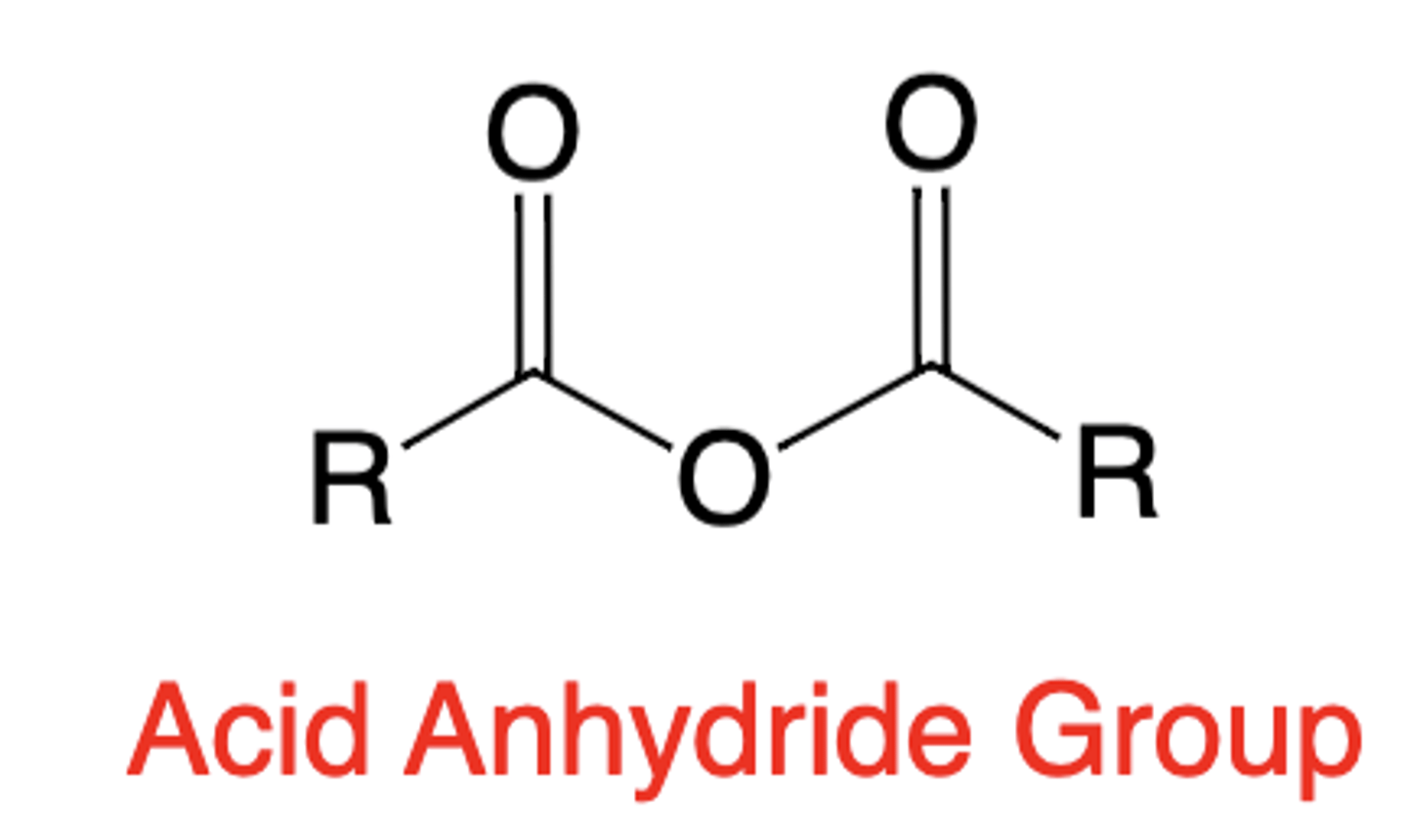
Anhydride group structure
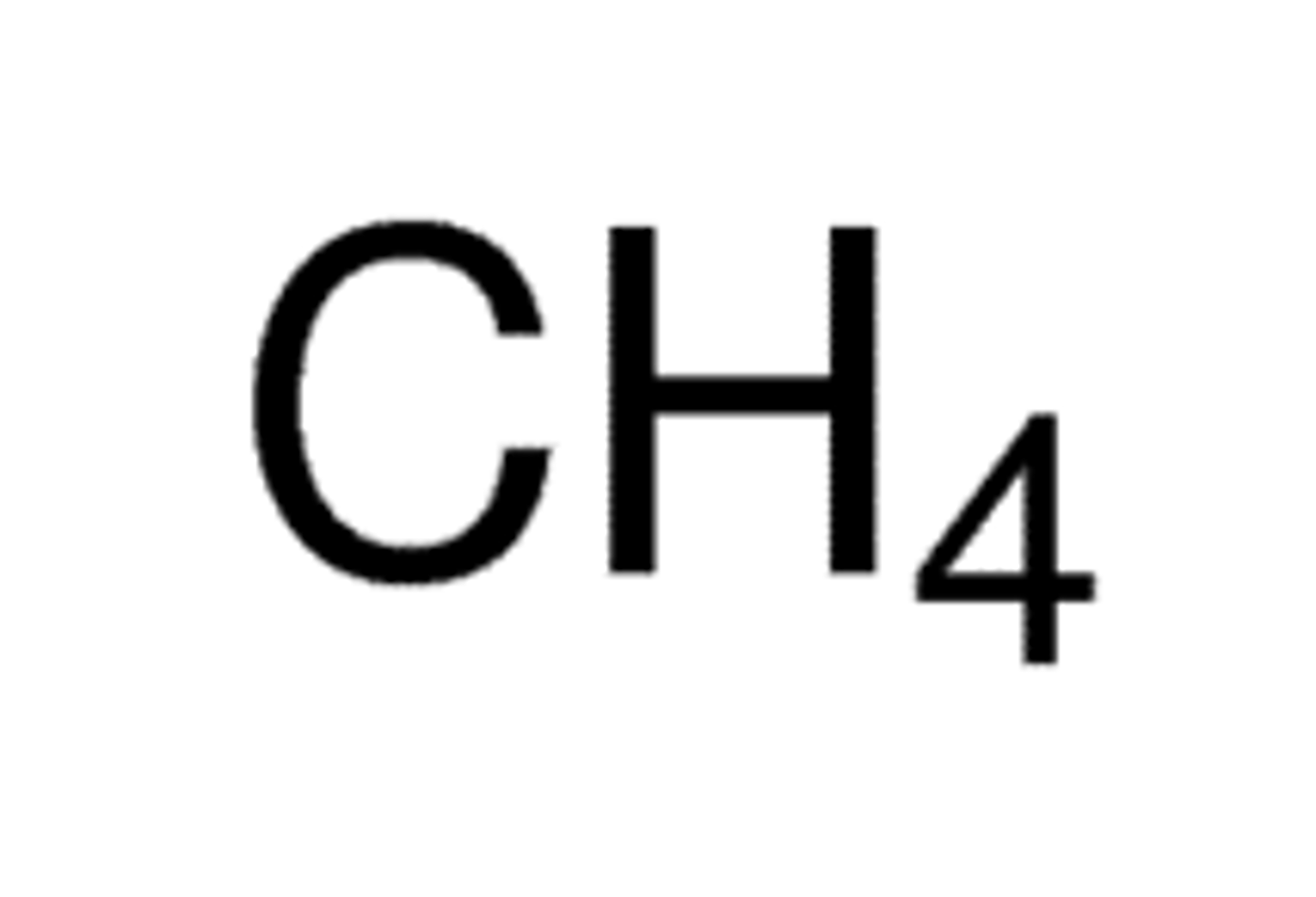
Methane structure
Ethane structure
Propane structure
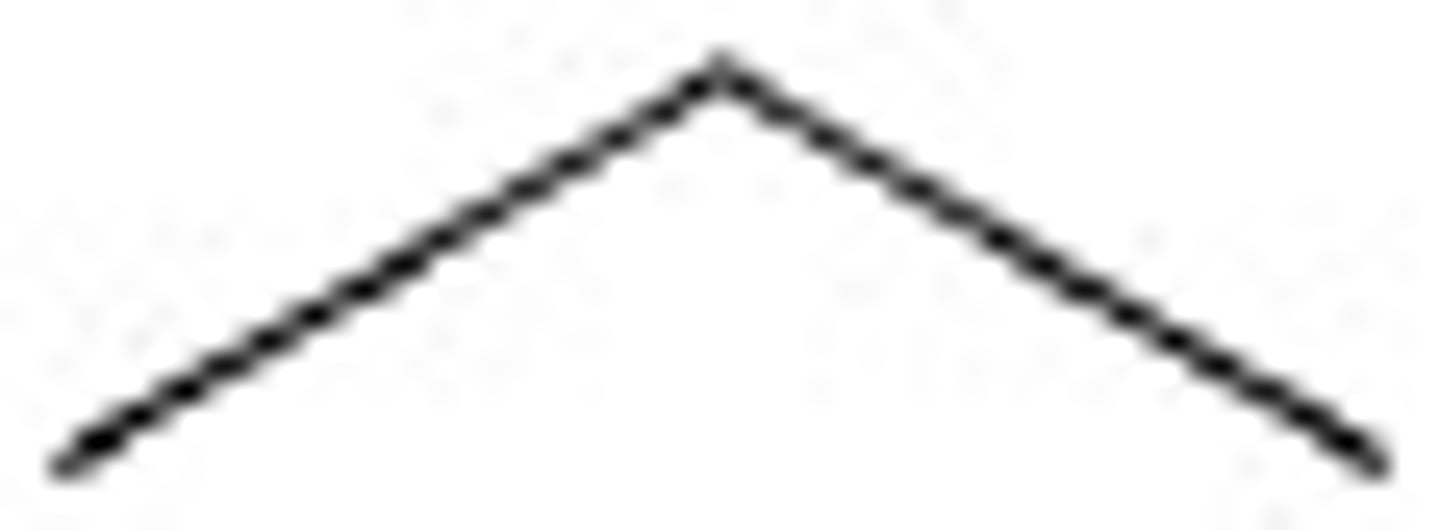
Butane structure

Pentane structure

Hexane structure
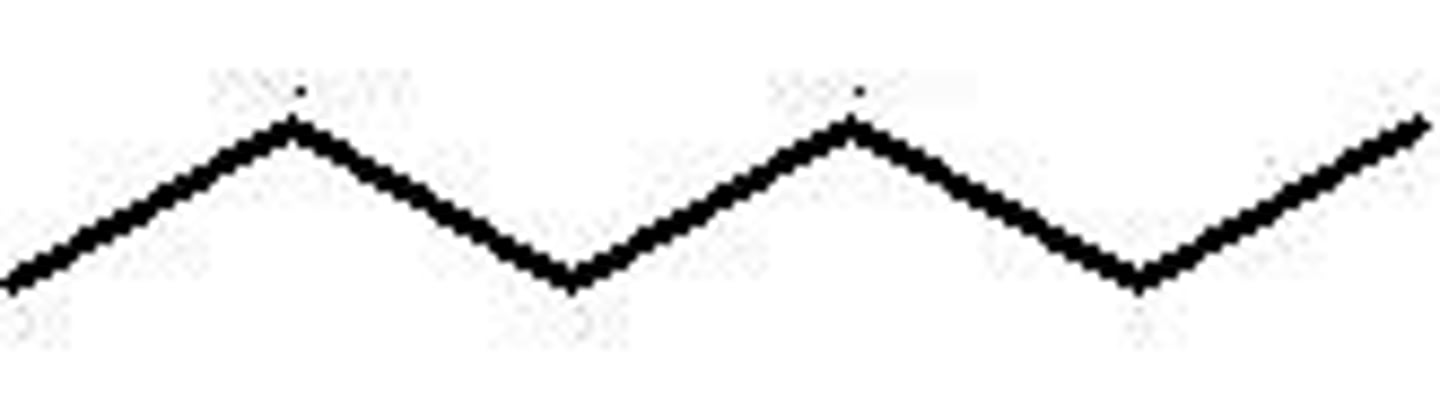
Heptane structure
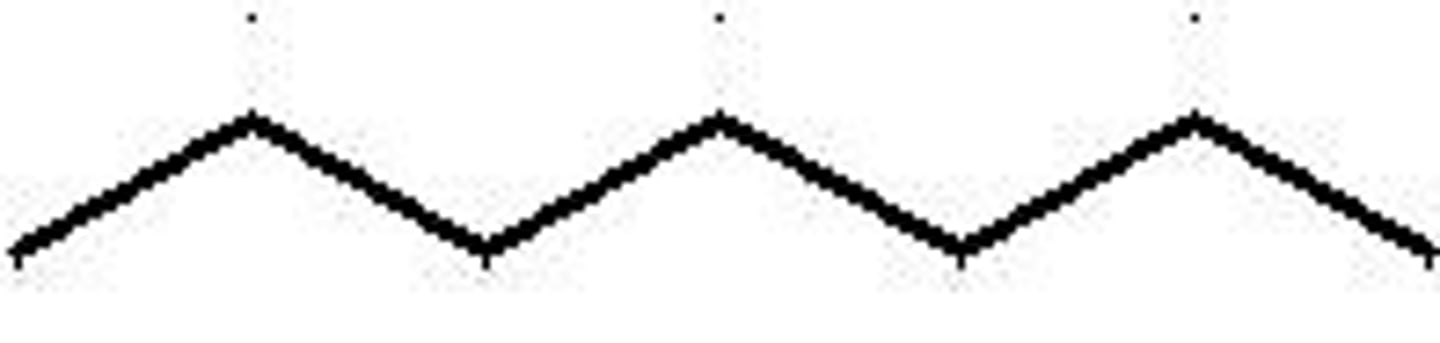
Octane structure
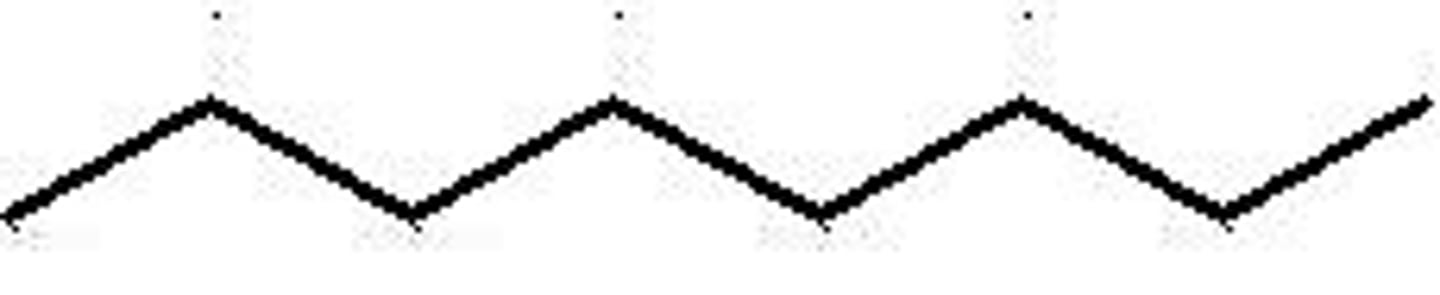
Nonane structure

Decane structure
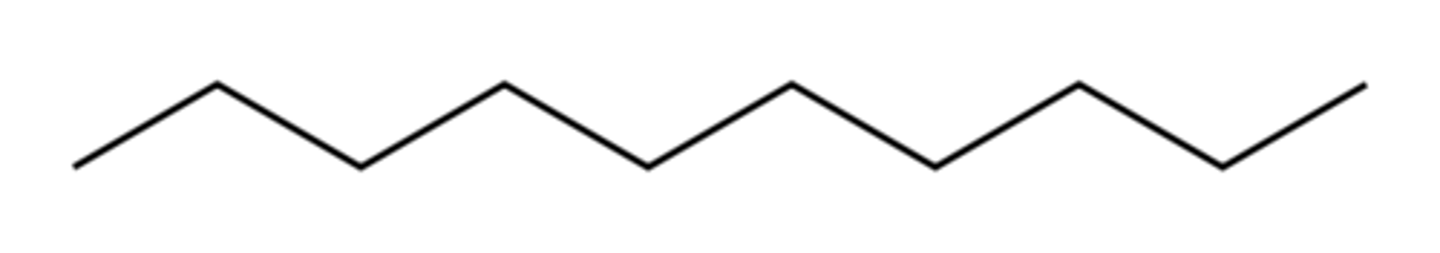
-ane structures...
have no double bonds present
-ene structures...
have a double bond present
iso- structures:
branched versions of the same compound
Isomers:
same molecular formula
Structural isomers:
different bonding arrangements of the same compound
cyclo- structures:
ringed versions of the functional groups
Trans- structures:
alkyl groups are on opposite sides of the double bond
Cis- structures:
alkyl groups are on the same sides of the double bond
Conjugation meaning:
Double bonds are alternating on the structure
Conformations:
-eclipsed
-staggered
Eclipsed conformation:
-high energy
-not optimal
Staggered conformation:
-lower energy
-optimal
Resonance structure:
one of the two or more equally valid structures of a molecule or polyatomic ion