Unit 3 – CHEMISTRY OF PROTEINS
1/88
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
89 Terms
(nitrogen) (protein) (proteios)
When scientists began studying nutrition in the early 19th century, they quickly discovered that natural
products containing (_________) were very essential for the survival of animals. This class of compound was
then coined as (_______) by the Swedish chemist Jacob Berzelius in 1939 which was derived from the Greek
word “(_______)” which means of first importance.
(15%) (50%)
Proteins are the most important macromolecule in the body because it plats a lot of important physiological
functions. It accounts for (_____) of total cell’s mass and for almost (___) of its dry weight.
(unbranched polymers) (amino acids) (peptide bonds)
Proteins are naturally occurring (______________) (long chain) of (________) connected
together by (________).
(native conformation) (hemoglobin) (amino acid composition)
The Structure-Function Relationship of Proteins
Proteins are large molecules with more than 50 amino acid
residues. As a result, they can take on various shapes or
conformations. Among all of these nearly infinite possibilities of
conformation, there is a specific structure of a specific protein that
will have a biological function. This particular structure is known as
its (__________).
As an example, consider (________). It is a protein in the blood
that transports oxygen from the lungs to the tissues. It cannot
function normally if it is not in its (1)(_________).
It must also be noted that this specific native conformation of a
protein is highly influenced by its (_______________).
(denaturation)
Changes in a protein’s native conformation is known as (_________). Because a protein's function is related to its structure, (1)(_________) can result in a loss of function.
(amino acids) (residue) (hydrolyzed) (free amino acids) (asparagine) (threonine) (cheese) (sweet)
Proteins are polymers of (_________), with each amino acid residue joined to its neighbor by a specific
type of covalent bond. (The term “(______)” reflects the loss of the elements of water when one amino acid
is joined to another.) Proteins can be broken down (________) to their constituent amino acids by a variety
of methods, and the earliest studies of proteins naturally focused on the (___________) derived from
them. Twenty different amino acids are commonly found in proteins. The first to be discovered was
(__________), in 1806. The last of the 20 to be found, (________), was not identified until 1938. All the amino
acids have trivial or common names, in some cases derived from the source from which they were first
isolated. Asparagine was first found in asparagus, and glutamate in wheat gluten; tyrosine was first isolated from cheese (its name is derived from the Greek tyros, “(_______)”); and glycine (Greek glykos, “(______)”) was
so named because of its sweet taste.
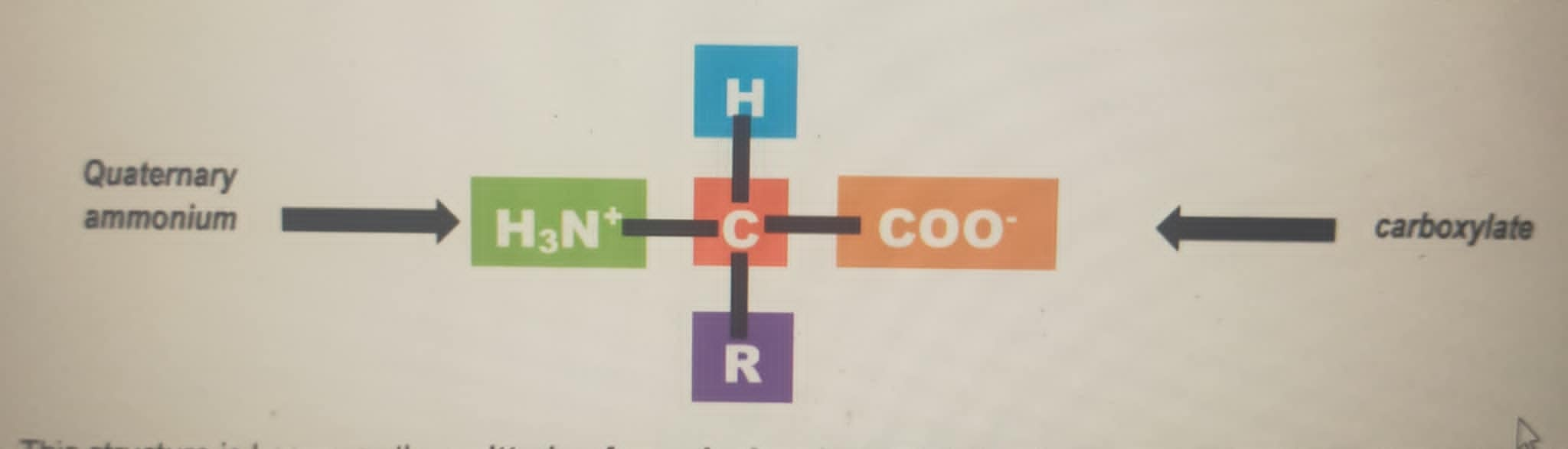
An amino acid is a compound containing a carboxyl (-COOH) group and amino (-NH2) group at the
same time. There are more than 300 naturally occurring amino acids, but only 20 of them are found in
humans. These are known as the Standard Amino Acids (SAA).
These Standard Amino Acids are α-amino acids because they have a carboxyl group and a primary
amino group attached in the same carbon, known as the α-carbon (See Figure below). The sole exception
is proline, as it has secondary amino group, although for uniformity we will also refer proline as an α-
amino acid.
(four) (α-carbon) (stereogenic centers)
The 20 Standard Amino Acids differ in the structure of their side chains, and, therefore, distinguishes them
from each other.
Enantiomers of Standard Amino Acids
If you noticed in the general structure of the Standard Amino Acids, there
are (____) different group around the (______).
This means that the α-carbons of the Standard Amino Acids are
(___________), except glycine since the side chain of glycine is H
resulting to 2 identical hydrogens in its α-carbon (See Figure on the right).
(stereogenic centers) (enantiomers – D) (L.)
Because 19 of the Standard Amino Acids have (____________), they also exist as (___________) or (___).
Fischer projection
To represent the D and L enantiomers of the Standard Amino Acids, we use the (___________) like in
monosaccharides. The Fischer projection of the D and L Standard Amino Acids
(amino group (-NH2) (L amino acids) (D amino acids) (bacterial cell walls) (peptide antibiotics)
The location of the (__________) of the α-carbon of a Standard Amino Acid in its Fischer projection
determines its type of enantiomer. For (________), the amino group of the α-carbon is directed to the left. For (__________), the amino group of the α-carbon is directed to the right. In nature and in proteins, the Standard Amino Acids are L isomers. D-Amino acid residues have been
found only in a few, generally small peptides, including some peptides of (___________) and certain (_____________).
(L stereoisomers) (asymmetric) (stereospecific)
It is remarkable that virtually all amino acid residues in proteins are (___________). When chiral
compounds are formed by ordinary chemical reactions, the result is a racemic mixture of D and L isomers,
which are difficult for a chemist to distinguish and separate. But to a living system, D and L isomers are as
different as the right hand and the left. The formation of stable, repeating substructures in proteins generally
requires that their constituent amino acids be of one stereochemical series. Cells are able to specifically
synthesize the L isomers of amino acids because the active sites of enzymes are (__________), causing the
reactions they catalyze to be (____________).
(three-letter code or one-letter code)
The standard amino acids are referred by their common names. These names are often abbreviated as
(_________/_________)
(nonpolar & hydrophobic) (highly polar & hydrophilic)
Because the standard amino acids are distinguished from each other by their side chains, we can classify
them according to the characteristics of their side chains. The most common way is based on polarity of
side chains, or tendency to interact with water at biological pH (near pH 7.0). The polarity of the R groups
varies widely, from (_______&_______) (water-insoluble) to (______&______) (water-soluble). The table below lists some of the common properties of the 20 standard amino acids, and the
figure that follows illustrates their structures.
(Nonpolar, Aliphatic R Groups) (alanine, valine, leucine, and isoleucine) (Glycine) (Methionine) (Proline)
(________________). The R groups in this class of amino acids are nonpolar and hydrophobic.
The side chains of (_______, _______, _______, _______) tend to cluster together within proteins,
stabilizing protein structure by means of hydrophobic interactions. (______) has the simplest structure.
Although it is formally nonpolar, it’s very small side chain makes no real contribution to hydrophobic interactions. (_______), one of the two sulfur-containing amino acids, has a non-polar thioether group in
its side chain. (_____) has an aliphatic side chain with a distinctive cyclic structure. The secondary amino
(imino) group of proline residues is held in a rigid conformation that reduces the structural flexibility of
polypeptide regions containing proline.
(Aromatic R Groups) (Phenylalanine, tyrosine, and tryptophan) (hydrogen bonds) (tyrosine hydroxyl group) (nitrogen) (tryptophan indole ring)
(______________). (_______________________________), with their aromatic side chains, are
relatively nonpolar (hydrophobic). All can participate in hydrophobic interactions. The hydroxyl group of tyrosine can form (__________), and it is an important functional group in some enzymes. Tyrosine and
tryptophan are significantly more polar than phenylalanine, because of the (_____________) and the
(_______) of the (_____________).
(ultraviolet light) (280 nm) (serine, threonine, cysteine, asparagine,) (glutamine) (hydroxyl groups) (sulfhydryl group) (amide groups)
Tryptophan and tyrosine, and to a much lesser extent phenylalanine, absorb (__________). This accounts
for the characteristic strong absorbance of light by most proteins at a wavelength of (________), a property
exploited by researchers in the characterization of proteins.
Polar, Uncharged R Groups. The R groups of these amino acids are more soluble in water, or more
hydrophilic, than those of the nonpolar amino acids, because they contain functional groups that form
hydrogen bonds with water. This class of amino acids includes (______________)
and glutamine. The polarity of serine and threonine is contributed by their (_________); that of cysteine
by its (__________); and that of asparagine and glutamine by their (______).
(acid or base) (cystine) (disulfide bond)
Asparagine and glutamine are the amides of two other
amino acids also found in proteins, aspartate and
glutamate, respectively, to which asparagine and glutamine
are easily hydrolyzed by (________). Cysteine is readily
oxidized to form a covalently linked dimeric amino acid
called (______), in which two cysteine molecules or residues
are joined by a (________) (See Figure at the side). The
disulfide-linked residues are strongly hydrophobic
(nonpolar). Disulfide bonds play a special role in the
structures of many proteins by forming co- valent links
between parts of a protein molecule or be- tween two
different polypeptide chains.
(Positively Charged (Basic) R Groups) (7.0) (lysine) (guanidino group) (imidazole group) (Histidine)
(________________________). The most hydrophilic R groups are those that are either positively
or negatively charged. The amino acids in which the R groups have significant positive charge at pH (___)
are (_______), which has a second primary amino group at the � position on it aliphatic chain; arginine, which
has a positively charged (___________); and histidine, which has an (________). (_______) is the
only common amino acid having an ionizable side chain with a pKa near neutrality. In many enzyme-
catalyzed reactions, a His residue facilitates the reaction by serving as a proton donor/acceptor.
(Negatively Charged (Acidic) R Groups)
(__________________). The two amino acids having R groups with a net negative charge
at pH 7.0 are aspartate and glutamate, each of which has a second carboxyl group.
(4-hydroxyproline) (5-hydroxylysine) (6-N-Methyllysine) (y-carboxyglutamate) (desmosine)
Some Uncommon Amino Acids
In addition to the 20 common amino acids, proteins may contain residues created by modification of
common residues already incorporated into a polypeptide (See figure below). Among these uncommon
amino acids are (____________), a derivative of proline, and (__________), derived from lysine. The
former is found in plant cell wall proteins, and both are found in collagen, a fibrous protein of connective
tissues. (_____________) is a constituent of myosin, a contractile protein of muscle. Another important
uncommon amino acid is (__________), found in the blood-clotting protein prothrombin and in
certain other proteins that bind Ca2+ as part of their biological function. More complex is (__________), a
derivative of four Lys residues, which is found in the fibrous protein elastin.
(Selenocysteine) (selenium)
(___________) is a special case. This rare amino acid residue is introduced during protein synthesis rather
than created through a postsynthetic modification. It contains (________) rather than the sulfur of cysteine.
Actually, derived from serine, selenocysteine is a constituent of just a few known proteins.
(300) (Ornithine and citrulline)
Some (_____) additional amino acids have been found in cells. They have a variety of functions but are not
constituents of proteins. (______________) deserve special note because they are key intermediates
(metabolites) in the biosynthesis of arginine and in the urea cycle.
(Essential amino acids) (10) (Phenylalanine, Valine, Threonine, Tryptophan, Isoleucine, Methionine, Histidine, Arginine, Leucine, and Lysine)
(____________) are Standard Amino Acids
that the human body cannot adequately
synthesize and must, therefore, be obtained from
dietary sources. There are (__) essential amino
acids necessary for normal growth of a child.
These amino acids can be easily memorized easily
using the mnemonic PVT. TIM HALL which stands
for: (________, _______, _______, ______, ______, ________, ________, ______, ________, ________)
(Arginine) (nonessential amino acids) (conditionally essential amino acids) (human milk) (infant formula milk)
(_______) is only essential for infants for normal
growth, but (_______________________) as
they grow into adulthood. Infants that are born
prematurely cannot make sufficient quantities of
some nonessential amino acids and these amino
acids become (________)
until the baby matures. In this situation the
conditionally essential amino acids must be obtained
through diet. The (________) and (____________) contain adequate amounts of these conditionally essential amino acids.
complete dietary protein
(high-quality protein)
A (________________)
is a protein that contains all of the essential amino
acids in adequate amounts as the body needs them.
Proteins from animal sources are usually complete
dietary protein. Example, casein from milk and
albumin from eggs are considered complete dietary
proteins.
(incomplete dietary protein) (limiting amino acid) (Gelatin)
An (_____________) is a protein that does
not contain in adequate amounts, relative to the
body’s needs, of one or more of the essential amino
acids. The essential amino acid that is missing or
present in an inadequate amount in an incomplete
dietary protein is known as a (______________).
(______) is an incomplete dietary protein having
tryptophan as its limiting amino acid.
(lysine, methionine, and tryptophan) (Soyprotein) (complementary dietary protein)
Protein from plant sources are generally incomplete
dietary proteins, with three common limiting amino
acids (________________). (___________) is the only common plant protein that is
considered complete dietary protein. However, mix of plant proteins generally provide complete dietary
protein, such as in the case of rice and beans. Proteins from rice and beans when eaten together are
known (__________________)
(amphoteric substance) (amphoteric)
Recall that an (___________________) is a substance that can act as an acid in basic environment and can
act as a base in an acidic environment. Amino acids are (___________).
) (–COOH) (–NH2)
How is amphoterism possible in amino acids?
The Standard Amino Acids have both carboxyl group (-COOH) and amino group (-NH2) in one molecule.
We learned in organic chemistry that (______) is an acidic group while (_____) is a basic group.
(carboxyl group (-COOH) (carboxylate)
At physiological pH (near neutral), (_____________) has the tendency to lose a proton (H+),
producing a negatively charged conjugate base known as (________).
(carboxyl group (-COOH) (amino group (-NH2) (quaternary ammonium ion) (deprotonated) (protonated)
At physiological pH (near neutral), (______________) has the tendency to lose a proton (H+),
producing a negatively charged conjugate base known as carboxylate.
RCOOH à RCOO- + H+
carboxylate ion
In the same manner, (_______________) have the tendency to accept a proton (H+), producing a positively
charged conjugate acid known as (__________________).
R – NH2 + H+ à R – NH3+
quaternary ammonium ion
Because of this acid-base property of the carboxyl and amino groups, in aqueous solutions, the –COOH of
the Standard Amino Acids is (_________) while the –NH2 is (___________). We can characterize this as an
intramolecular acid-base reaction. When this happens, the Standard Amino Acid assumes the
(zwitterion form) (zwitterion or dipolar) (zwitterion)
This structure is known as the (___________) of a Standard Amino Acid. A (______________) ion is a
molecule having a positive charge on one side and negative charge on the other. Take note that while a
has charged groups it is neutral molecule because the number of groups with positive charge
is equal to the number of groups with negative charge resulting to zero net charge. In solid state, the
standard amino acids exist as (___________).
(acidic) (low pH or basic high pH) (increased)
Structural Changes of the Zwitterion at Varying pH
The zwitterion structure of a Standard Amino Acid changes when the pH of the solution containing the
Standard Amino Acid is changed from neutral to either (_________________). When the solution is made acidic, the H+ concentration is (________) by adding an acid, such as HCl.
When the solution of a Standard Amino Acid is made acidic (abundant H+), the carboxylate portion of the
zwitterion is protonated (accepts H+) to form a positively charged species.
(deprotonated)
When the solution is made basic, the OH- concentration is increased by adding a base, such as NaOH.
When the solution of a Standard Amino Acid is made basic (abundant OH-), the quaternary ammonium
portion of the zwitterion is (__________) (loses H+) to form a negatively charged species.
(zwitterion, negative
ion, and positive ion)
Thus in aqueous solutions, 3 different form of Standard Amino Acid forms can exist – (_______, ________, _______). These forms are in equilibrium with each other, and the dominant form is determined
by the pH of the solution.
(gain or loses) (four)
The discussion above assumes that the side chain (R group) of a Standard Amino Acid remains unchanged
in solution as the pH is varied. That is only the case for amino acids whose side chains do not ionize. There
are other amino acids whose side chain can acquire a charge because it contains an amino group or a
carboxyl group that can, respectively, (___________) a proton as the pH of the solution is varied. Because of
the extra site that can be protonated or deprotonated, acidic and basic amino acids have (____) charged forms
in the solution.
(aspartic acid )
Notice that the zwitterion for (__________) is predominant in a moderately acidic solution. That
is the characteristic of amino acids having negatively charged side chains.
• Notice as well that the carboxyl group of the α-carbon was first to deprotonate than the carboxyl
group in the side chain. How do we know this? Looking at the table in Page 4, the carboxyl group
of the α-carbon has a lower pKa value than the carboxyl group in the side chain. This means that
the carboxyl of the α-carbon is a stronger acid and has the more tendency to deprotonate first.
(Isoelectric point) (pI)
An important pH value, relative to the various forms an amino acid can have in solution, is the pH of at
which it exists primarily in its zwitterion form. This pH is known as the (1) (____________) given the symbol
(__). At (1), almost all amino acid molecules in a solution (> 99%) exist as zwitterions.
(Electrophoresis) (Cathode) (anode)
Separation of Amino Acids by Electrophoresis (Application of Isoelectric Point)
(____________) is a common method for separating charged molecules in an electric field. paper is used as a matrix where separation take place. In gel electrophoresis, cross-linked
gelatin-like substance is used as matrix. For the purpose of our discussion, we will use paper electrophoresis.
The procedure of paper electrophoresis is summarized below:
1. A small amount of sample solution containing amino acids is placed in the center of a solid matrix. Both
ends of the matrix is soaked in ionic buffer solution having a specific pH.
2. An electric voltage is applied at the electrodes immersed in the buffer. (_______) refers to the negative
electrode, while (______) refers to the positive electrode. The amino acids in the sample will then migrate to
the electrodes, and the direction of migration is determined by its form in the buffer.
(peptides) (polypeptides)
We now turn to polymers of amino acids, the (________). Biologically occurring (________) range in size
from small to very large, consisting of two or three to thousands of linked amino acid residues. Our focus is
on the fundamental chemical properties of these polymers.
(peptide bond) (linkage)
Two amino acid molecules can be covalently joined through a substituted amide linkage, termed a (_______), to yield a dipeptide. Such a (______) is formed by removal of the elements of water (dehydration) from
the �-carboxyl group of one amino acid and the �-amino group of another:
(Peptide bond formation)
(_______________) is an example of a condensation reaction, a common class of reactions in living
cells.
(tripeptide) (tetrapeptides) (pentapeptides) (oligopeptide) (polypeptide) (below) (higher)
Three amino acids can be joined by two peptide bonds to form a (_______); similarly, amino acids can be
linked to form (___________), (_________), and so forth. When a few amino acids are joined in this
fashion, the structure is called an (__________). When many amino acids are joined, the product is called
a (_________). Proteins may have thousands of amino acid residues. Although the terms “protein” and
“polypeptide” are sometimes used interchangeably, molecules referred to as polypeptides generally have
molecular weights (_____) 10,000, and those called proteins have (_____) molecular weights.
(residue) (α-amino group) (α-carboxyl group) (N-terminal) (C-terminal residue)
General Structural Feature of a Peptide Chain
As already noted, an amino acid unit in a peptide is often called a (______) (the part left over after losing a
hydro- gen atom from its amino group and the hydroxyl moiety from its carboxyl group). A peptide chain
has directionality because its ends are different – an (_______) is present at one end, and an (_________) at the other. The amino acid residue with a free α-amino group is the (_______)
residue; the residue at the other end, which has a free α-carboxyl group, is the (___________).
(peptide backbone) (acceptor) (proline) (donor)
The (___________) of a peptide or a protein consists of the repeated sequence –N–Cα– C–, where N
stands for the amino or amide nitrogen, the Cα is α-carbon atom of an amino acid residue, and the final
C is the carbonyl carbon of the amino acid, which in turn is linked to the amide N of the next amino acid
down the line.
is rich in hydrogen-bonding potential. Each residue contains a carbonyl group
(C=O) which is a good hydrogen bond (________), and, with the exception of (______), an NH group, which
is a good hydrogen bond (_____). These groups will interact with the groups in the side chains of some
amino acid residues stabilizing the overall structure of the protein.
Oxytocin and Vasopressin
Oxytocin and Vasopressin
These are peptide hormones that are both produced in the pituitary gland. Each hormone is a
nonapeptide, with six of the amino acid residues held in the form of a loop by a disulfide bond
formed from the interaction of 2 cysteine residues. Structurally, these nanopeptides differ in the
amino acid present in positions 3 and 8 of the peptide chain. In both structures, an amino group
replaces the C-terminal single-bonded oxygen atom. Oxytocin regulates uterine contraction and lactation.
Vasopressin, also called as antidiuretic hormone (ADH), regulates the excretion of water
in kidneys and affects blood pressure.
(Enkephalins)
(_________)
These are pentapeptide neurotransmitters produced by the brain itself that bind at receptor sites in
the brain to reduce pain.
The two well–known enkephalins are Met-enkephalin and Leu-enkephalin (sequence shown below),
whose structures differ only at the C-terminal end of the peptide; this amino acid difference is
incorporated in their names.
Met-enkephalin: Tyr-Gly-Gly-Phe-Met
Leu-enkephalin: Tyr-Gly-Gly-Phe-Leu
The painkillers morphine and codeine can bind at the same receptor sites in the brain as the naturally
occurring (1) (__________), and thus can reduce pain. The pain relief of morphine and codeine lasts long; they are not affected by the enzymes in the brain that normally hydrolyzes
the peptide bonds in enkephalins.
(Glutathione) (glutamic acid)
(________)
This is a tripeptide with the sequence Glu-Cys-Gly which is present in significant concentrations in
most cells which serves as an antioxidant, protecting cellular component against oxidizing agents,
such as peroxides and superoxides (highly reactive species of oxygen often generated within the
cell in response to bacterial invasion.
The structure is a bit odd. The (________) is bonded to cysteine through the side-
chain carboxyl group rather than through its α-carbon carboxyl group.
(protein) (Monomeric proteins) (Multimeric proteins)
The term (______) is reserved for polypeptides with a large number of amino acid residues, usually more
than 40 residues. It may contain only one or more than one polypeptide chain. For this reason,
it can be classified as:
1. (___________) – contain only one polypeptide chain (protein subunit)
2. (___________) – contain more than one protein subunits. The protein subunits in a
multimeric protein may be all identical to each other or completely different from each other. An
example of a multimeric protein is insulin, composed of 2 protein subunits that are different from
each other.
Proteins may also contain non-amino acid groups known as prosthetic groups. Thus, proteins can also
be classified as:
1. Simple proteins – only amino acid is present
2. Conjugated proteins – contain amino acids and prosthetic groups
(Monomeric proteins) (Multimeric proteins) (Simple proteins) (Conjugated proteins)
it can be classified as:
1. (___________) – contain only one polypeptide chain (protein subunit)
2. (___________) – contain more than one protein subunits. The protein subunits in a
(2) (_________) may be all identical to each other or completely different from each other. An
example of a multimeric protein is insulin, composed of 2 protein subunits that are different from
each other.
Proteins may also contain non-amino acid groups known as prosthetic groups. Thus, proteins can also
be classified as:
1. (_____________)– only amino acid is present
2. (_____________) – contain amino acids and prosthetic groups
(conformations) (biological functions) (native conformations) (primary, secondary, tertiary, and quaternary).
Due to the enormous size of a protein, it can assume many different (____________) (three-dimensional
structure). Of these many structures, one or a few have (_____________). These are called (____________). The (3) (____________) of a protein has no predictable repeating structure, but it is not
random. Meaning to say, although there are no specific rules for determining how a protein would look, the
same native conformation is found in all molecules of a given protein.
Describing a protein’s overall three-dimensional structure is complex; thus, we define it in terms of four
levels of structure – (_______, _______, ________, ______)
Primary structure
(__________) of a protein is the order at
which the amino acid residues are covalently
linked together. For example, Leu-Gly-Thr-Val-
Arg-Asp-His has a different primary structure from
the peptide Val-His-Asp-Leu-Gly-Arg-Thr, even
though both have the same number and kinds of
amino acids.
Secondary structure
(_________) of a protein is the localized
arrangement of the atoms in the peptide
backbone. This arrangement of the peptide
backbone is maintained by hydrogen bonds.
There are a lot of types of secondary structure of
proteins that will later examine in this lecture
notes.
(Tertiary structure)
(____________) of a protein is the over-all
three-dimensional arrangements of all the atoms
im a polypeptide chain, including the side-chains
and prosthetic groups. Several covalent and non-
covalent forces are responsible for maintaining a
protein’s (1) (__________).
(subunits) (Quaternary structure)
As mentioned previously, a protein may consist
multiple polypeptide chains called (_______).
(________) is the arrangement of
these subunits with respect to one another. It i exists only
for those proteins having multiple subunits
(multimeric proteins). It is mediated by non-covalent interactions. All proteins have until tertiary structure, but not all
proteins have (2) (___________).
(amino acid sequence) (primary structure) (genes)
The (_________) of a protein, its (__________), determines its three-dimensional structure,
which, in turn, determine its functions. The sequence of amino acids in a protein’s primary structure is
decided by the (_____).
(Insulin)
(____) is necessary for proper utilization of carbohydrates.
(Human insulin)
(________) consists of two chains
having a total of 51 amino acids. The two chains are connected by disulfide bonds
(the same function) (human insulin)
Despite the slight differences in structure, all of these insulins perform (________) and even
can be used by humans. However, none of the other three is quite as effective as (__________). Another factor showing the effect of substituting one amino acid for another is that
sometimes patients become allergic to, say, bovine insulin but can switch to hog or sheep insulin
without experiencing an allergic reaction.
(hemoglobin) (sickle-cell anemia) (Human hemoglobin) (HbS) (valine residue in HbS)
some small changes in amino acid sequence make a great deal
of difference. One of the most striking demonstrations for this is found in the (____________) associated
with (__________).
(____________) is composed of four subunits, 2 α chains and 2 β chains, which sums up to a
total of 574 amino acid residues Some people develop a genetic defect resulting to a slightly different kind of hemoglobin in their
blood, called (____). This hemoglobin differs from the normal type only in the β chains and only in one
position on these 2 chains: The glutamic acid in the sixth position of normal HB is replaced by a (_______________).
(sickle cell anemia) (sickled cells)
This change affects only a single amino acid residue in a molecule containing 574 amino acid
residues, yet it is enough to produce a very serious disease, (_______). The name is based
from the fact that red blood cells containing HbS assume a sickle shape. The
(_________) tend to become trapped in small blood vessels, cutting off circulation and thereby
causing organ damage.
The next level of protein structure is the secondary structure, which refers to regular localized
arrangement of polypeptide backbone of the protein. Recall, that the backbone just refers to the
polypeptide chain apart from the side chain – so all we mean here is that secondary structure does not
involve R group atoms. The secondary structure is stabilized by hydrogen bond between the carbonyl
group (C=O) and the N-H group in the peptide backbone.
(the α helix and the β pleated sheet).
There are two commonly occurring secondary structures in proteins – (_________&________)
These 2 are periodic structures, meaning their features repeat in regular intervals.
(Helix)
The α (_____) is helical (spiral) repeating structure of a polypeptide chain. The shape is maintained by hydrogen bond between the carbonyl (–C=O) of one amino acid and an N-H of another
amino acid located four residues further along the polypeptide chain, that is between the C=O of the
1st amino acid and the N-H of the 5th amino acid along the chain. In turn, one turn of the (1) (_______) comprises
3.6 amino acid residues (3 N-Cα-C, 1 C=O, and 1 N-H).
(extend outward) (pitch)
In an α helix, all side chains (_________) of the spiral. The linear distance between corresponding points
on successive turns, called the (_____), is 5.4 Å (0.54 nm). The helical conformation is very stable because
it allows for a linear arrangement of the atoms involved in the hydrogen bonds, which gives the bonds
maximum strength. The hydrogen bonds are parallel to the direction of the polypeptide.
(Proline) (electrostatic repulsion) (bulky groups) (Steric repulsion)
The following are factors that can disrupt the α helix:
1. Presence of the amino acid (________)
is known as a helix breaker. It creates a bend in the backbone once it its incorporated in a
peptide, thus it destabilizes the helical conformation.
2. Presence of proximate similarly charged groups
Amino acids with similarly charged side chains that are near to each other cause electrostatic
repulsion destabilizing the helical conformation. Thus, there is a disruption in the α helix when
lysine and arginine are too close to each other in a polypeptide because these amino acids both
have positively charged side chains and would repel from each other. This is also true for glutamic
acid and aspartic acid.
3. Presence of (_________)
Whenever amino acids with bulky side chains are close to each other in a polypeptide chain, it can
cause crowding which results in (_________), which also weakens the stability of the
helical conformation.
(β Pleated Sheet)
(____________)
In this type of secondary structure, the peptide backbone is almost fully extended. The hydrogen bonds
can be formed between different parts of a single chain that is doubled back on itself (intrachain bonds)
or between different chains (interchain bonds).
(antiparallel β sheet) (parallel β sheet) (parallel arrangement)
Adjacent strands in a β sheet can run in opposite
directions (_____________) or in the same
direction (_________). In the antiparallel
arrangement, the NH group and the C=O group of each
amino acid are respectively hydrogen bonded to the
CO group and the NH group of a partner on the
adjacent chain. In the (______________), the hydrogen-bonding
scheme is slightly more complicated. For each amino
acid, the NH group is hydrogen bonded to the CO
group of one amino acid on the adjacent strand,
whereas the CO group is hydrogen bonded to the NH
group on the amino acid two residues farther along
the chain
(β Turns) (antiparallel β sheet) (180o)
(_______)
In globular proteins, which have a compact folded structure, nearly one-third of the amino acid residues are
in turns or loops where the polypeptide chain reverses direction. These are the
connecting elements that link successive runs of α helix or β conformation. Particularly common are (1) (______)
that connect the ends of two adjacent segments of an (_______). The structure is a (____) turn
involving four amino acid residues, with the carbonyl oxygen of the first residue forming a hydrogen bond
with the amino-group hydrogen of the fourth. The peptide groups of the central two residues do not
participate in any interresidue hydrogen bonding.
(Gly and Pro) (the surface)
(____/___) residues often occur in β turns, the former because
it is small and flexible, the latter because peptide bonds involving
the imino nitrogen of proline readily assume the cis configuration, a form that is particularly amenable to a
tight turn. Of the several types of β turns, the two shown in the
figure above are the most common. Beta turns are often found
near (______) of a protein, where the peptide groups of the
central two amino acid residues in the turn can hydrogen-bond
with water. Considerably less common is the β turn, a three-
residue turn with a hydrogen bond between the first and third
residues.
(disulfide bond). (cysteine residue)
Recall, the 2 cysteine residues can react to form the dimer cystine (________) When a
cysteine residue is in one chain and another cysteine residue is in another chain (or in another part of the same chain), formation of a (1) (________) provides a covalent linkage that binds together the
two chains or the two parts of the same chain.
(Side-chain hydrogen bonding)
(______________)
This interaction can occur between polar groups on side chains or between side chains and the
peptide backbone.
(Electrostatic Attraction (Salt Bridge)
(______________)
This can occur between 2 amino acid residues with ionized side chains – that is, between an acidic
amino acid (-COO-) and a basic amino acid (–NH3+). The two are held together by simple ion-ion
attraction.
(Hydrophobic Interaction) (hydrophobic interactions)
(________________)
In aqueous solution, globular proteins usually turn their polar groups outward, toward the aqueous
solvent, and their nonpolar groups inward, away from the water molecules. The nonpolar groups
prefer to interact with each other, excluding water from these regions. The result is a series of
(________________).
Although this type of interaction is weaker than hydrogen bonding or salt bridges, it usually acts
over large surface areas, so that the interactions are collectively strong enough to stabilize a loop
or some other tertiary structure formation.
(Metal Ion Coordination) (same charge)
(________________)
Two side chains with the (______) would normally repel each other, but they can also be linked
via a metal ion. For example, two glutamic acid side chains (-COO-) would both be attracted to a
magnesium ion (Mg2+), forming a bridge. This is one reason that the human body requires certain
trace minerals—they are necessary components of proteins.
(oxygen-storage and transport) (Hydrogen bonds, electrostatic attractions, and hydrophobic interactions)
The three-dimensional conformation of a protein is the result of the interplay of all the stabilizing forces. Not
every protein necessarily exhibits all possible structural features of the kinds just described. For instance,
there are no disulfide bridges in myoglobin and hemoglobin, which are (______________)
proteins and classic examples of protein structure, but they both contain Fe(II) ions as part of a prosthetic
group. In contrast, the enzymes trypsin and chymotrypsin do not contain complexed metal ions, but they
do have disulfide bridges. (______, _________, _______, _______)
occur in most proteins.
(nuclear magnetic resonance (NMR) spectroscopy).
The protein’s three-dimensional structure is determined by X-ray crystallography and, recently, by (____________________________)
(quaternary structure) (multimeric protein)
The final level of protein structure is the (_____________), and it
refers to the association of the different polypeptide chains of a
(___________). The association of polypeptide chains can serve a variety
of functions. Many multisubunit proteins have regulatory roles; the
binding of small molecules may affect the interaction between subunits,
causing large changes in the protein’s activity in response to small
changes in the concentration of substrate or regulatory molecules.
(multimer) (oligomer) (protomer)
A multisubunit protein is also referred to as a (________). Multimeric proteins can have from two to hundreds
of subunits. A multimer with just a few subunits is often called an (_________). If a multimer is composed of a
number of nonidentical subunits, the overall structure of the protein can be asymmetric and quite
complicated. However, most multimers have identical subunits or repeating groups of nonidentical subunits,
usually in symmetric arrangements. The repeating structural unit in such a multimeric protein, whether it is
a single subunit or a group of subunits, is called a (________). The subunits interact with one another non-
covalently via electrostatic attractions, hydrogen bonds, and hydrophobic interactions.
(Allosteric Proteins) (allosteric)
(_____________)
As a result of non-covalent interactions in a proteins quaternary structure, subtle changes in structure at
one site on a protein molecule may cause drastic changes in properties at a distant site. Proteins that exhibit
this property are called (_______).
The heme group is the same in both myoglobin and hemoglobin. One molecule of myoglobin binds to one
oxygen molecule. Four molecules of oxygen can bind to one hemoglobin molecule. Both hemoglobin and
myoglobin bind oxygen reversibly, but the binding of oxygen to hemoglobin exhibits positive cooperativity,
whereas oxygen binding to myoglobin does not.
(Positive cooperativity) (hyperbolic) (sigmoidal) (cooperative binding)
(_____________) means that when one oxygen molecule is bound, it becomes easier for the next
molecule to bind. A graph of the oxygen-binding properties of hemoglobin and myoglobin is one of the best
ways to illustrate this point.
When the degree of saturation of myoglobin with oxygen is plotted against oxygen pressure, a steady rise
is observed until complete saturation is approached and the curve levels off. The oxygen-binding curve of
myoglobin is thus said to be (________). In contrast, the oxygen-binding curve for hemoglobin is
(________). This shape indicates that the binding of the first oxygen molecule facilitates the binding of the
second oxygen, which facilitates the binding of the third oxygen, which in turn facilitates the binding of the
fourth oxygen. This is precisely what is meant by the term “(______________).”
(Myoglobin) (hemoglobin)
The two types of behavior are related to the functions of these proteins.
(__________) has the function of oxygen storage in muscle. It must
bind strongly to oxygen at very low pressures, and it is 50% saturated
at a partial pressure of oxygen of 1 torr. The function of (___________) is
oxygen transport, and it must be able both to bind strongly to
oxygen and to release oxygen easily, depending upon conditions.
In the alveoli of lungs (where hemoglobin must bind oxygen for
transport to the tissues), the oxygen pressure is 100 torr. At this
pressure, hemoglobin is 100% saturated with oxygen. In the capillaries
running through active muscles, the pressure of oxygen is 20 torr,
corresponding to less than 50% saturation of hemoglobin, which occurs
at 26 torr. In other words, hemoglobin gives up oxygen easily in
capillaries, where the need for oxygen is great.
(Hydrolysis)
(_________) of proteins causes disruption of the peptide bonds causing liberation of free amino acids.
This can be carried out in the presence of strong acids, strong bases, and enzymes (proteases).
-can be complete or partial.
(Denaturation)
(___________), on the other hand, causes disruption (unfolding) of a protein’s three-dimensional structure
due to the breakdown of the non-covalent interactions. Since the three-dimensional structure of proteins is
very much related to their functions, (1) (_______), therefore, causes loss of biological activity.
(Heat)
Denaturation can be reversible or irreversible. Irreversible denaturation results in coagulation and
precipitation. The following are agents that can cause denaturation of proteins:
1. (____) cleaves hydrogen bonds, so boiling a protein solution destroys the α-helical and β-pleated
sheet structure. In collagen, the triple helixes disappear upon boiling, and the molecules have a
largely random-coil conformation in the denatured state, which is gelatin. In other proteins,
especially globular proteins, heat causes the unfolding of the polypeptide chains; because of
subsequent intermolecular protein–protein interactions, coagulation then takes place. That is what
happens when we boil an egg.
(6 M aqueous urea) globular proteins. (Surface active agents (detergents) (Acids, bases, and salts) (2-mercaptoethanol)
2. Addition of denaturing chemicals, such as (___________), also breaks hydrogen bonds and
causes the unfolding of globular proteins.
3. (______________________) change protein conformation by opening the hydrophobic
regions.
4. (______, _______, _____) affect both salt bridges and hydrogen bonds.
5. Reducing agents, such as (_______________) can break disulfide bonds, reducing to -SH groups.
The processes of permanent waving and straightening of curly hair are examples of the latter effect.
The protein keratin, which makes up human hair, contains a high percentage of disulfide bonds.
These bonds are primarily responsible for the shape of the hair, whether straight or curly. In either
permanent waving or straightening, the hair is first treated with a reducing agent that cleaves some
of the disulfide bonds. This treatment allows the molecules to lose their rigid orientations and
become more flexible. The hair is then set into the desired shape, using curlers or rollers, and an
oxidizing agent is applied. The oxidizing agent reverses the preceding reaction, forming new
disulfide bonds, which now hold the molecules together in the desired positions.
(Heavy metal ions) (Alcohol)
6. (__________) (for example, Pb2+, Hg2+, and Cd2+) also denature protein by attacking the –SH
groups. They form salt bridges, as in –SHg2+–S-
. This very feature is taken advantage of in the
antidote for heavy metal poisoning: raw egg whites and milk. The egg and milk proteins are
denatured by the metal ions, forming insoluble precipitates in the stomach. These must be pumped
out or removed by inducing vomiting. In this way, the poisonous metal ions are removed from the
body. If the antidote is not pumped out of the stomach, the digestive enzymes would degrade the
proteins and release the poisonous heavy metal ions, which would then be absorbed into the
bloodstream.
7. (________) also denatures proteins, coagulating them. This process is used in sterilizing the skin before
injections. At a concentration of 70%, ethanol penetrates bacteria and kills them by coagulating their
proteins, whereas 95% alcohol denatures only surface proteins.