STRUCTURAL EFFECTS ON SOLUBILITY, MELTING POINT, AND BOILING POINT
1/105
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
106 Terms
INTERMOLECULAR FORCES
attractive forces between molecules
• van der Waals
• Hydrogen bonding
• Ion-dipole interaction
INTERMOLECULAR FORCES types 3
INTERMOLECULAR FORCES
All _ are electrostatic, involving attractions
between positive and negative species.
electrostatic attraction
interaction between positive and negative charge
INTERMOLECULAR FORCES
are the forces that hold molecules in a substance
INTERMOLECULAR FORCES
Weaker than intramolecular forces
INTERMOLECULAR FORCES
Determine the state of matter (solid/liquid/gas) and their physical properties such as melting/boiling point etc.
INTERMOLECULAR FORCES
Attractive forces
INTERMOLECULAR FORCES
Categorized into dipole-dipole forces, London dispersion forces and hydrogen bonding forces
INTRAMOLECULAR FORCES
are the forces that hold atoms in a molecule
INTRAMOLECULAR FORCES
Stronger than inte
rmolecular forces
INTRAMOLECULAR FORCES
Determine chemical behavior of a substance
INTRAMOLECULAR FORCES
Chemical bonds
INTRAMOLECULAR FORCES
Categorized into covalent, ionic and metal bonds
Strong intramolecular attraction (covalent bond)
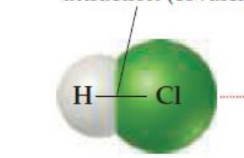
Weak intermolecular attraction

intramolecular force/attraction (covalent bond)
within one molecule of hcl there’s an _
intramolecular force
chemical bonds
intermolecular force
between molecules there’s an _
London
Debye
Keesom
Van der Waals forces 3 types
London
dispersion forces (induced dipole-induced dipole)
Debye
dipole-induced dipole
Keesom
dipole-dipole
INTRAMOLECULAR FORCES
ability to particaipte in a chemical reaction
INTERMOLECULAR FORCES
ARE MORE IMPORTANT/RELEVANT
LONDON DISPERSION FORCE
a.k.a. induced dipole-induced dipole interactions
LONDON DISPERSION FORCE
attractive force that arises as a result of temporary dipoles induced in
atoms or molecules
LONDON DISPERSION FORCE
present in all substances (polar or nonpolar)
LONDON DISPERSION FORCE
weakest
LONDON DISPERSION FORCE
DISPERSION FORCE/DIPOLE-INDUCED DIPOLE
DIPOLE
SEPARATION OF CHARGES/ EXISTENCE OF 2 POLES WITH OPPOSITE CHARGES
LONDON DISPERSION FORCES
An instantaneous dipole (due to movement of electrons)
LONDON DISPERSION FORCES
momentary dipoles/temporary dipoles
nonpolar
even in _ substances, there can be temporary/momentary.instantaneous dipoles
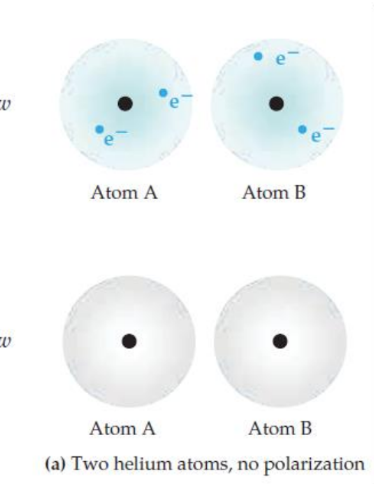
In this initial state, the electrons in both atoms are evenly distributed around the nucleus. There's no separation of charge, meaning neither atom has a positive or negative pole.
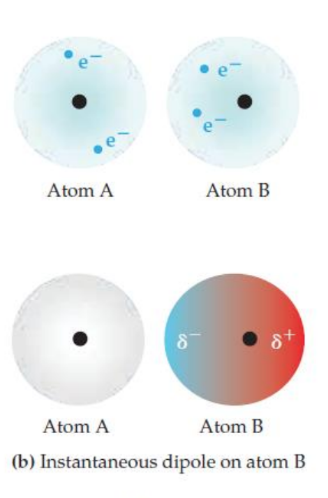
Electrons are constantly moving. At any given instant, the electron distribution might become uneven in one atom (Atom B in this case). This creates a temporary, instantaneous dipole – one side becomes slightly negative (δ-) due to a momentary excess of electrons, and the other side becomes slightly positive (δ+).
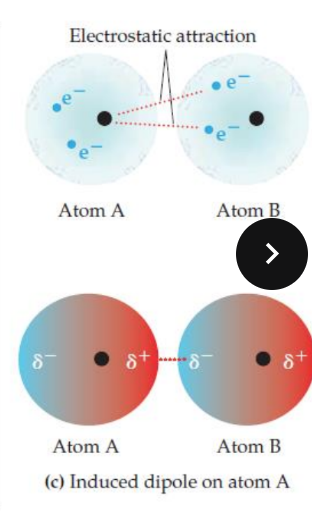
The temporary dipole in Atom B affects the electron distribution in Atom A. The positive pole (δ+) of Atom B attracts the electrons in Atom A, causing them to shift and create an induced dipole in Atom A. This results in an electrostatic attraction between the positive pole of Atom A and the negative pole of Atom B.
repel
like charges will
induction
_ of dipole is due to the polarization of adjacent atom for molecules
LONDON DISPERSION FORCE
significant only when molecules are close together
LONDON DISPERSION FORCE
depends on area of contact: greater area of contact, stronger London
dispersion forces
greater
LONDON DISPERSION FORCE
depends on area of contact: _ area of contact, stronger London
dispersion forces
stronger
LONDON DISPERSION FORCE
depends on area of contact: greater area of contact, _ London
dispersion forces
Increase
_ in MW increases London dispersion force
increases
Increase in MW _ London dispersion force
Branching
_ decreases London dispersion force
increase MW
how to increase area of contact?
chain
longer _. longer area of contact
branching
_ decreases area of contact/london dispersion forces
KEESOM FORCE
a.k.a. dipole-dipole interaction
KEESOM FORCE
electrostatic attraction between the partially positive end of one
molecule and the partially negative end of a neighboring molecule
KEESOM FORCE
present in polar compounds (only)
KEESOM FORCE
stronger than London dispersion force
polar-polar
permanent dipole
non-polar
momentary dipoles
attractive dipole-dipole interaction (red); organized
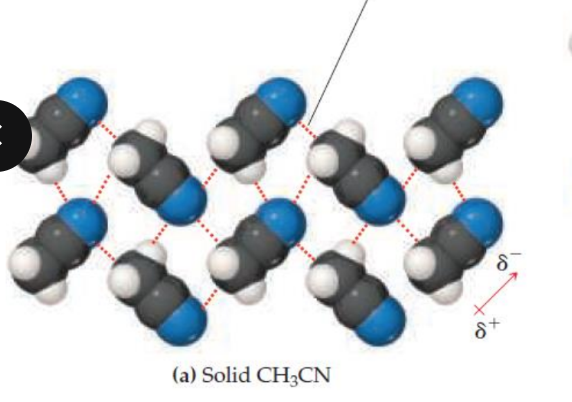
repulsive dipole-dipole interaction (blue); randomly oriented
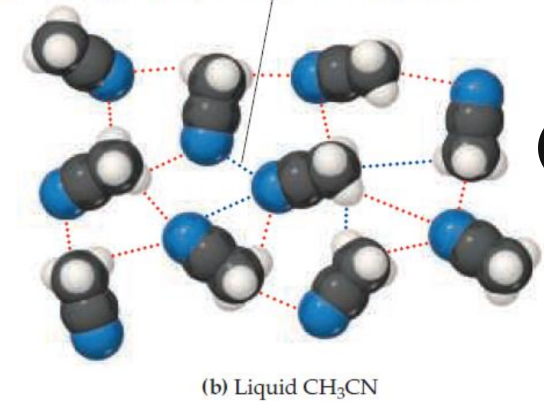
KEESOM FORCE
depends on polarity
polarity
KEESOM FORCE
Increase in _ increases dipole-dipole interaction
dipole-dipole interaction
KEESOM FORCE
Increase in polarity increases _
LONDON & KEESOM
both are van der Waals
Keesom
Even though _ is stronger, London is more significant in
determining the bulk properties of matter.
London
Even though Keesom is stronger, _ is more significant in
determining the bulk properties of matter.
bulk
Even though Keesom is stronger, London is more significant in
determining the _ properties of matter.
HYDROGEN BONDING
electrostatic attraction between a hydrogen attached to an
electronegative atom (N, O, F) and a lone pair of an electronegative
atom (N, O, F) in another molecule
HYDROGEN BONDING
a strong type of dipole-dipole interaction
N O F
HYDROGEN BONDING hydrogen attached to an
electronegative atoms (3)
HYDROGEN BONDING
depends on the electronegativity of atom interacting with hydrogen
HYDROGEN BONDING
special type of dipole-dipole; not classified under dipole-dipole; more impt contributor to the bulk properties
ION-DIPOLE INTERACTION
electrostatic attraction between an ion and a polar molecule
cation
_ with partially negative end of polar molecule
anion
_ with partially positive end of polar molecule
electrostatic attraction
H is partially positive; N, O, F are partially negative
ION-DIPOLE INTERACTION
present in ionic compounds dissolved in polar solvents
ION-DIPOLE INTERACTION
strongest
>
HF _ H2O - NH3
ion-dipole interaction
one is ionic; one is a polar compound
ION-DIPOLE INTERACTION
depends on ionic charge and polarity
covalent
in org chem, most compounds are not ionic they’re
ION-DIPOLE INTERACTION
The positively charged sodium ion (Na+) attracts the partially negative (δ-) oxygen ends of the water molecules. This aligns the water molecules around the sodium ion with their oxygen atoms pointing towards it.
ION-DIPOLE INTERACTION
The negatively charged chloride ion (Cl-) attracts the partially positive (δ+) hydrogen ends of the water molecules. This aligns the water molecules around the chloride ion with their hydrogen atoms pointing towards it.
van der waals and hyrogen bonding
most impt imf 2 in our discussion
Dispersion forces (London Dispersion Forces)
Weakest IMF.
Present in all substances.
Become stronger with larger molecules.
Examples: CH₄, Br₂.
Energies: 0.1-30 kJ/mol.
Dipole-dipole forces
Occur between polar molecules.
Stronger than dispersion forces.
Examples: CH₃F, HBr.
Energies: 2-15 kJ/mol.
Hydrogen bonding
A special type of dipole-dipole force.
Occurs when H is bonded to N, O, or F.
Relatively strong IMF.
Examples: NH₃, CH₃OH.
Energies: 10-40 kJ/mol.
Ion-dipole forces
Occur between ions and polar molecules.
Stronger than hydrogen bonding.
Examples: NaCl dissolved in H₂O.
Energies: >50 kJ/mol.
Ionic bonding
Not strictly an IMF, but a strong chemical bond.
Occurs between ions in ionic compounds.
Strongest interaction.
Examples: KBr, NH₄NO₃.
Energies: >150 kJ/mol.
Dispersion forces
occur in all molecules but are dominant in nonpolar molecules.
Dipole-dipole forces
exist in polar molecules without hydrogen bonding.
Hydrogen bonding
occurs when H is bonded to N, O, or F.
Ion-dipole forces
happen when ionic compounds dissolve in polar solvents.
Ionic bonding
is the strongest and occurs between fully charged ions.
Dispersion Forces (0.1–30 kJ/mol)
Present in all molecules and atoms (weakest interaction).
Dispersion Forces (0.1–30 kJ/mol)
Applies to: Atoms (Ne, Ar), Nonpolar molecules (BF₃, CH₄), Polar molecules (HCl, CH₃CN, H₂O, NH₃).
Dipole-Dipole Interactions (2–15 kJ/mol)
Occur in polar molecules without hydrogen bonding.
Dipole-Dipole Interactions (2–15 kJ/mol)
Applies to: Polar molecules without OH, NH, or HF groups (HCl, CH₃CN), and Polar molecules containing OH, NH, or HF groups (H₂O, NH₃).
Hydrogen Bonding (10–40 kJ/mol)
Strong interaction found in polar molecules containing OH, NH, or HF groups.
Hydrogen Bonding (10–40 kJ/mol)
Applies to: Molecules like H₂O and NH₃.
Ion-Dipole Interactions (>50 kJ/mol)
Strong force between ions and polar molecules.
Ion-Dipole Interactions (>50 kJ/mol)
Applies to: Ionic solids dissolved in polar liquids (e.g., NaCl in H₂O).