Chemistry CLEP (Khan Academy and Jeremy Krug)
1/109
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
110 Terms
How many questions and how much time do you get on the exam. What tools do you get?
75 questions in 90 minutes. TI-30 Calculator and uncolored periodic table
What is 1 mol (1 mole) equal too
Also known as Avogadro's number = 6.022 × 10²³ fundamental units.
When finding the mass of a compound like C6H12O6 what is the molar mass in terms of
grams/moles (g/mol)
Isotope
Referring to an element who has a differing number of neutrons from the original atom
Protons, neutrons and electrons on a diagram or periodic table
On a periodic table, the top number is the atomic number, or the number of protons. If you have an element like the one on the right, the top left number is the atomic mass (Protons + Neutrons). The bottom left is the number of protons. If there's a number on the top right, you do the protons minus that number to find the number of electrons. If there's no charge, protons = electrons

What's the purpose of a mass spectrometer
It gives you the relative abundance of each elements isotopes. If you want to know which element is represented by the diagram, you should find the average mass and look at the periodic table

Empirical formula
Think of it has a compound that has the lowest terms. For example, If you have Glucose, C6H12O6, the empirical formula is CH2O.
How do you determine an empirical formula
Too find an empirical formula, you can think of it as you having 100 grams of a substance. So 49.5% would be 49.5 grams. You then multiply by the molar mass of each element and the smallest one is what you divide each output by. Those are the subscripts.

Coulombs law
Permittivity of free space is 8.85×10-12
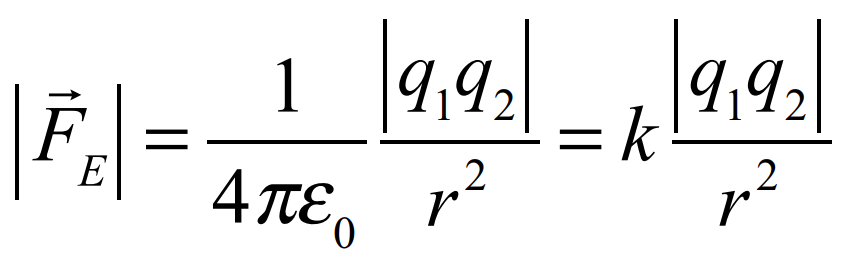
How to do Electron Configurations
You always start at hydrogen and go left to right. As you go through you keep count of the region your in and thats the superscript. For example, Borons electron configuration would be 1s22s22pWe omit superscripts of 1. This is Borons electron configuration because we went through 1s twice, 2s twice, and 2p once. The superscripts should add to the number of electrons the element has.

Ionization energy on the periodic table
Increases as you move towards the top right of the table, decreases as you go to the bottom left.
Atomic radius on the periodic table
As you go down on the table, atomic radius increases. As you go up, atomic radius decreases.
Electronegativity on the periodic table
A measure of how well an atom can attract an electron. If you go up on the periodic table, electronegativity increases. If you go to the right, electronegativity also increases.
Valence electrons on the periodic table
Valence electrons are located in the outermost occupied energy level of an atom and it can form chemical bonds
Electron Affinity
Increases as you go up and to the right

Covalent bond production
Happens when two nonmetals share electrons
Nonpolar covalent bond
When two nonmetals share electrons equally or almost equally
Polar covalent bond
When two nonmetals share electrons unequally
How do I know if electrons between nonmetals are shared equally or unequally.
If the difference in the electronegativity of the atoms is <0.5, that’s nonpolar covalent.
If the difference is >=0.5 thats polar covalent
(The atom with higher electronegativity would be the one stealing the electrons)
Ionic bonds
Bonds between metal and nonmetal.
Metallic Bonding
Electrons are able to move freely instead of being stuck to the nuclei from which they came.
Electrolytes
Conduct electricity when dissolved in water
FOR NUCLEAR CHEMISTRY
Lewis Electron Dot Diagrams
Use your periodic table for the valence electrons A method of arranging dots that represent valence electrons around the atomic symbol. Consider bonds when drawing them and that dash on the left indicates that they’re bonded.

Octet Rule
Most atoms are stable with 8 valence electrons.
What are some major exceptions to the octet rule
Hydrogen: Most stable with 2 electrons
Boron: Tends to be most stable with 6 electrons
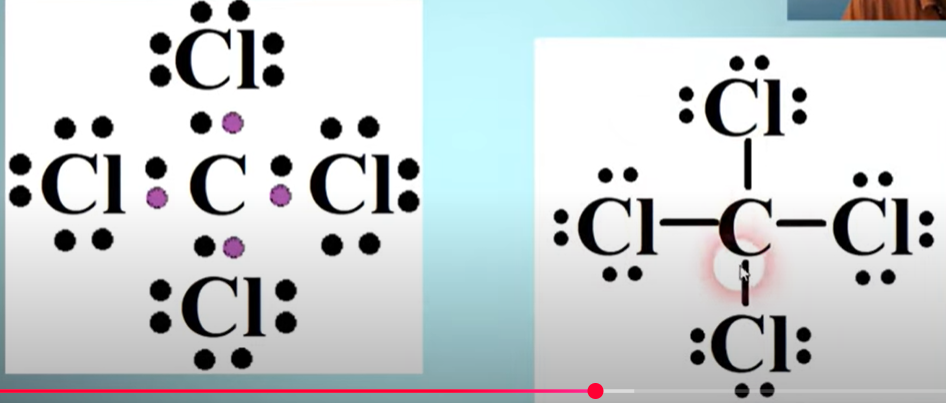
Example for Lewis Electron dot diagram for CCl4
The atom all by itself is in the middle. Its usually easier to start with the outside elements and assign their valence electrons. Then work your way in. Purple dots indicate carbons electrons. (Keep in mind of exceptions for the octet rule)
Example for Lewis Electron dot diagram for CO2
You have to orient the electrons in a way to where they all share 8 electrons. Since theres two parts where we share the electrons, theres 2 bonds. (Keep in mind of exceptions for the octet rule)

(Finish Unit 2) (Sigma and Pi Bonds)
Finishing Unit 2
Whats an Ideal Gas
Gases whose particles don’t interact with eachother and have no volume
Volume and pressure share what type of relationship (Boyle’s Law) What’s its equation
Inverse relationship
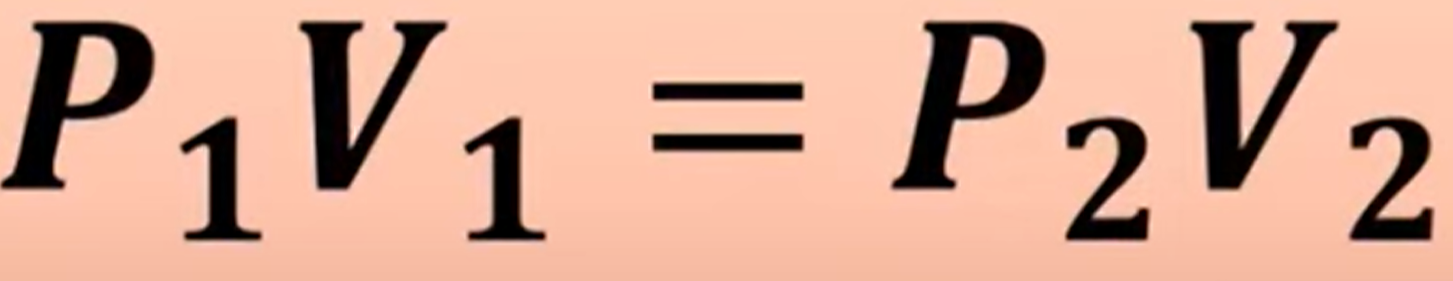
Volume and temperature share what type of relationship. (Charles Law) What’s its equation
Direct Relationship
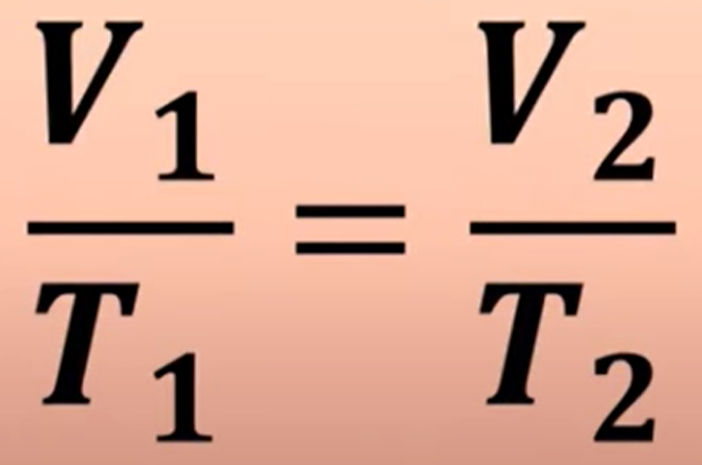
Pressure and temperature share what type of relationship. (Gay-Lussac’s Law) What’s its equation
Direct Relationship
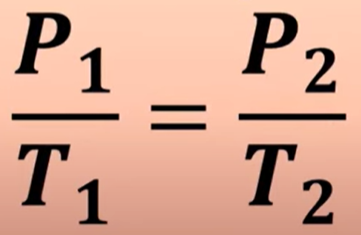
What’s the equation for the Combined Gas Law
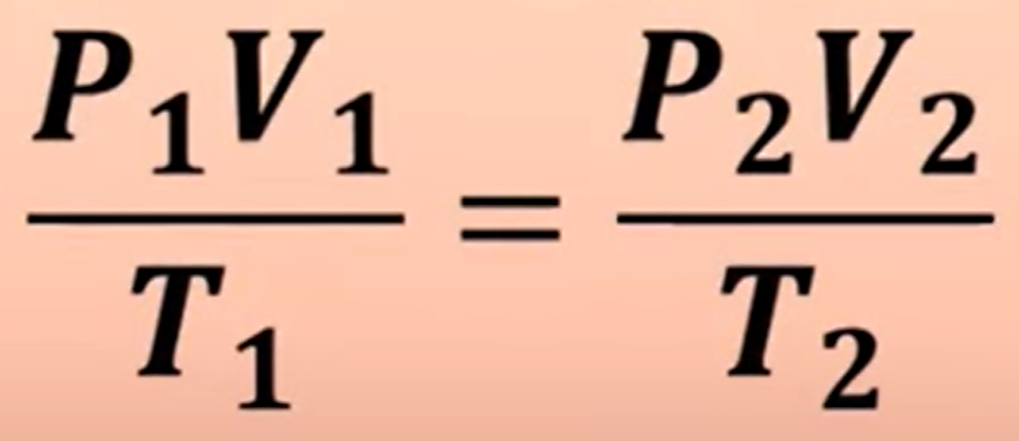
Volume and number of moles share what type of relationship (Avogrado’s Law)
Direct relationship
Ideal gas law
PV=nRT. T is temperature in Kelvin, R is the ideal gas constant, n is the number of moles, V is volume, and P is pressure
Conversion from Celsius to Kelvin
Kelvin = Celsius + 273.15
Depending on what your given in an ideal gas law problem, you need different ideal gas law constants. Name each constant with units.
R=8.314 J/(mol•K) = 0.08206 (L•atm)/(mol•K) = 62.36 (L•Torr)/(mol•K)
Conversion between atmospheres (atm), millimetre of Mercury (mm Hg), and Torr (Torr)
1 atm = 760 mm Hg = 760 Torr
Partial Pressure formula
P is pressure, and X is the moles of the gas divided by total moles. You can add pressures together
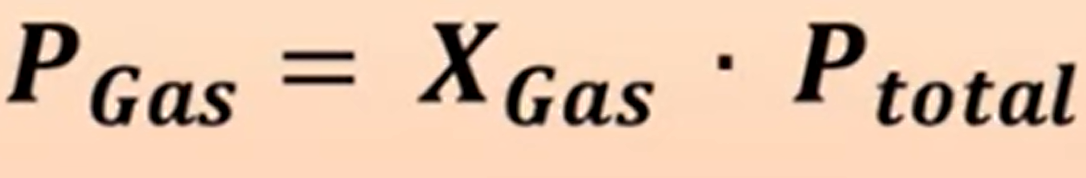
Boltzman Distribution Curve
Says average kinetic energy increases as temperature increases. Why is the area under each curve the same? Cause were talking about the same gas.

Grahams Law Equation
Rate of diffusion or effusion is inversely related to the square root of its molar mass. Note that the fractions do not coorespond

Mixtures
Materials that contain more than one substance
What are the two types of mixtures
Heterogeneous mixtures and Homogeneous mixtures
Heterogeneous mixtures
Individual parts are visible to the naked eye
Homogeneous mixtures
Components are mixed together uniformly. Cannot be seen by naked eye. (Also known as solution)
Dilution Equation
M1V1 = M2V2. M is molarity. V is volume
Dilute Solutions have what
Relatively low amount of solute
Concentrated Solutions have what
Relatively high amount of solute in the solution
Saturated Solutions
Concentrated solutions that have dissolved the maximum amount of solute for that temperature.
Supersaturated solutions
Concentrated solutions that have temporarily dissolved more than the maximum amount of solute for that temperature
What does aqueous mean
Dissolved in water
Ionic compounds
Metal and nonmetal. Cation and anion
Mathematical relationship for frequency and wavelength
C is the speed of light, lambda is wavelength and nu is frequency. C = 2.998×108 m/s

What’s the energy of a photon
h is plancks constant which is 6.626×10-34 J*s
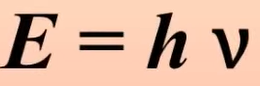
Solubility rules. Water is the universal solvent
1: All nitrates (NO3) are soluble
2: All group 1 ions + Ammonium (NH4) are soluble
3: All acetates are soluble (C2H3O2)
4: All chlorides, bromides, and iodides, and soluable, EXCEPT for compounds containing silver (Ag), lead (Pb), and Mercury (Hg)
5: All sulfates, SO4, are soluble except for silver, lead, mercury, calcium, strontium, and bromine compounds
Percent yield
(Actual/Theoretical)*100
Finishing Unit 4
Kinetics
Study of rates of chemical reactions
In a concentration V time graph, what's the rate of change of concentration of the substance

What is the general format of the rate law equation
K is a constant with appropriate units. K is known as the rate constant

What is the overall order of a reaction equal too
Adding all the exponents together
Plot concentration vs time. If the plot is a straight line, what order is the reaction
0th order. Rate = k[A]0 = k = slope.
Plot ln(concentration) vs time. If the plot is a straight line, what order is the reaction
1st order. Rate = k[A]. K is slope.
Plot the reciprocal of concentration vs time. If the plot is a straight line, what order is the reaction
2nd order. Rate = k[A]². K is slope.
For a 0th order reaction, what equation can be used for concentration throughout time

For a 1st order reaction, what equation can be used for concentration throughout time
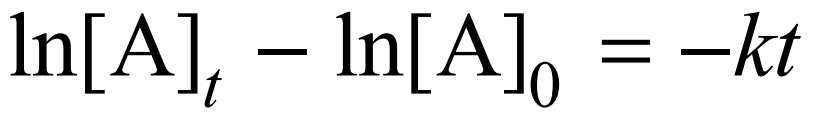
Half-life equation
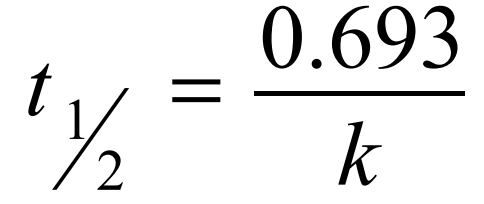
For a 2nd order reaction, what equation can be used for concentration throughout time
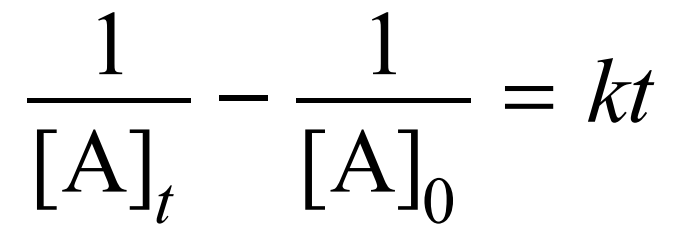
First law of Thermodynamics
Total energy of an isolated system is constant. Energy is not created or destroyed only transferred.
Enthalpy
Heat associated with the formation and breaking of chemical bonds
Exothermic process
Heat/Enthalpy is released
Endothermic process
Heat/Enthalpy is absorbed
How hot or cold something is known as temperature. This is also
A measure of the average kinetic energy of the particles
In an endothermic reaction, what is the enthalpy
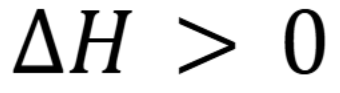
In an exothermic reaction, what is the enthalpy

Heat of a system
Where q the amount of heat absorbed in joules, m is the mass of the object in grams, c is the specific heat capacity of the material in joules/(gram*Celsius) and T is temperature in Celsius.
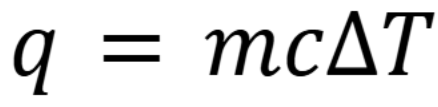
Equilibrium definition
The rate of the forward reaction and reverse reaction are equal
Equilibrium misconception
At equilibrium, the reactant concentrations are NOT necessarily equal to the product concentration.
Equilibrium Constant Equation
Products to there coefficients, divided by the reactants to their coefficients. Products over reactants. Equilibrium constants are unitless. Omit pure liquids and solids in equilibrium constant expressions. Lots of the time, the equilibrium constants are very large or very small.
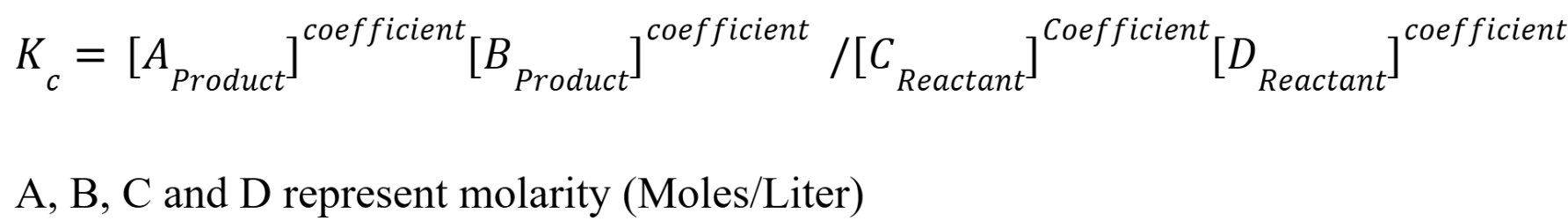
What does the equilibrium constant mean
If the equilibrium constant is large (>1) then there's lots of products, and not much reactant. If its small (<1) then there's lots of reactant and not much product
Formula for Molarity, M
Mol/L or Moles/Liters
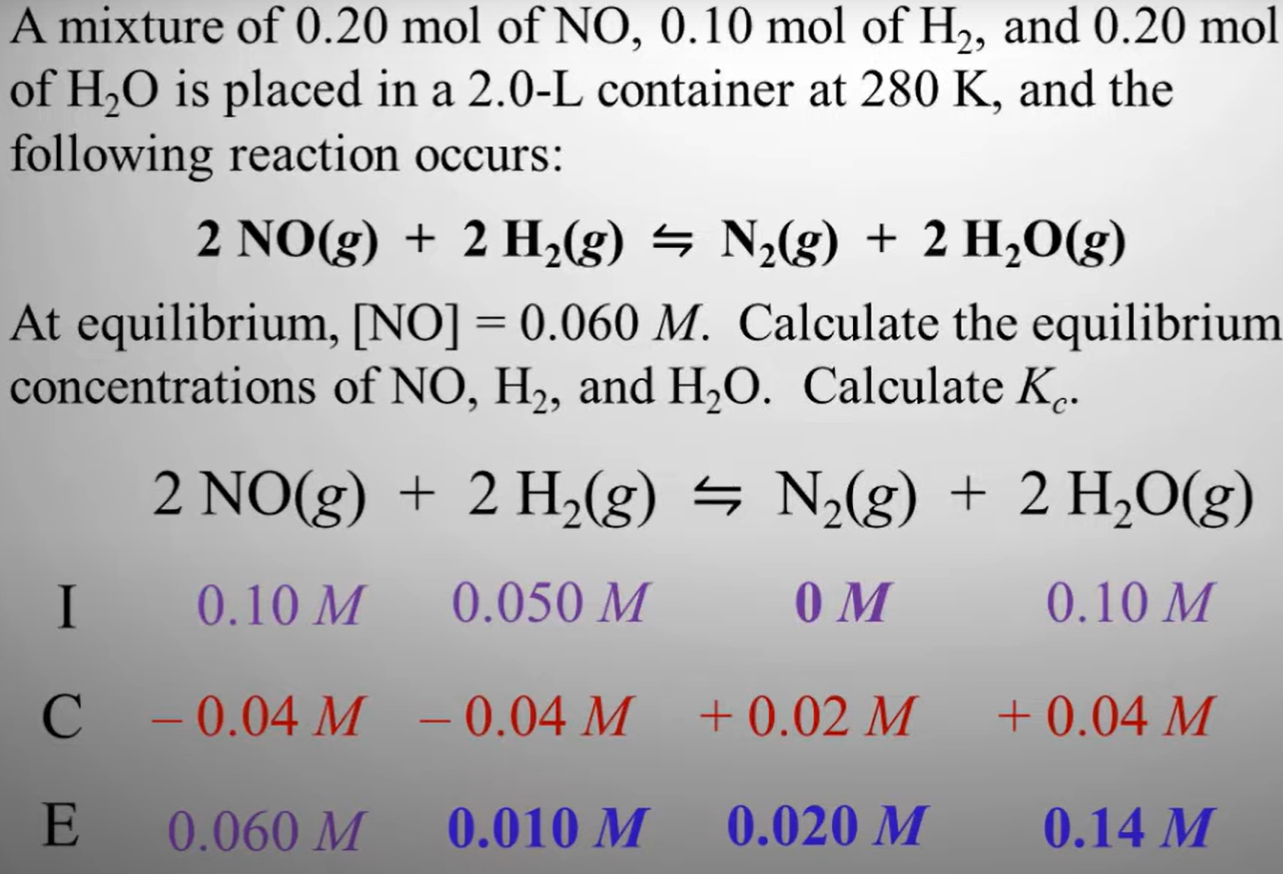
Example ICE problem
Purple indicates what we already know. I means initial concentration, C means change in concentration, E is equilibrium concentration. Apply Molarity = Mol/L. For the change in, do E - I because that's the final - initial. Since NO2 and H2 share the same mol to mol ratio (Same coefficients), they have the same change. Since the left side is taking away concentration, the left must add. N2 is adding 0.02 M because its half of 0.04 M and its mol to mol ratio of NO2 is half. Then find Kc.
What does the 5% rule say for equilibrium
You can omit subtraction in an equilibrium problem if the x value, divided by what your taking it away from, times 100 is less than 5%. So (X/the molarity)*100. This works for very small equilibrium constants
Le Chatelier's Principle
When a system at equilibrium is disturbed, the system will adjust too be back in equilibrium.
When a substance is added to one side of a reaction what happens
Causes an increase in concentration on the other side
When a substance is removed from one side of a reaction what happens
Causes an increase in concentration on the same side
Increasing the pressure (Decreasing the volume), shifts equilibrium where
Toward the side with fewer moles of gases
Lowering the temperature does what to an equilibrium reaction
Shifts the equilibrium reaction to the left
Increasing the temperature does what to an equilibrium reaction
Shifts the equilibrium reaction to the right
Continue 7.11
The strong acids
These are the only strong acids that exist.
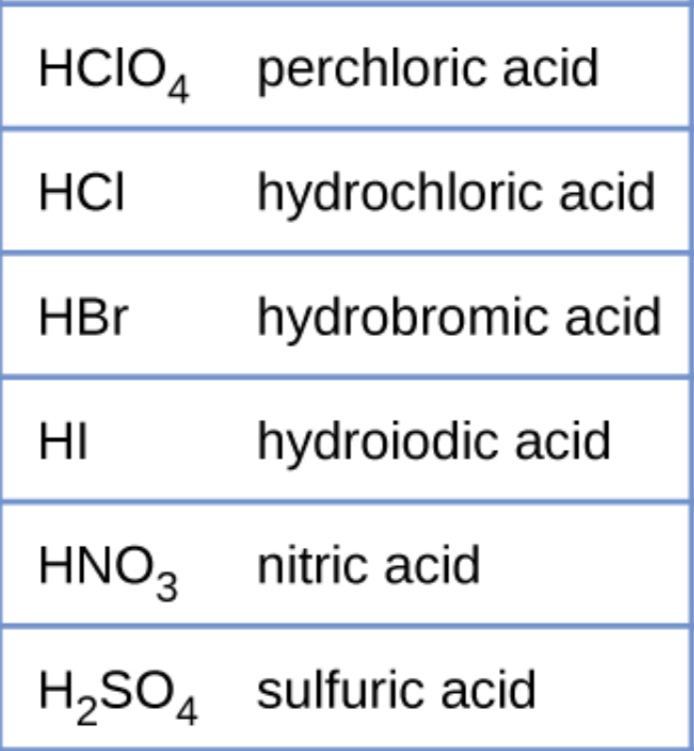
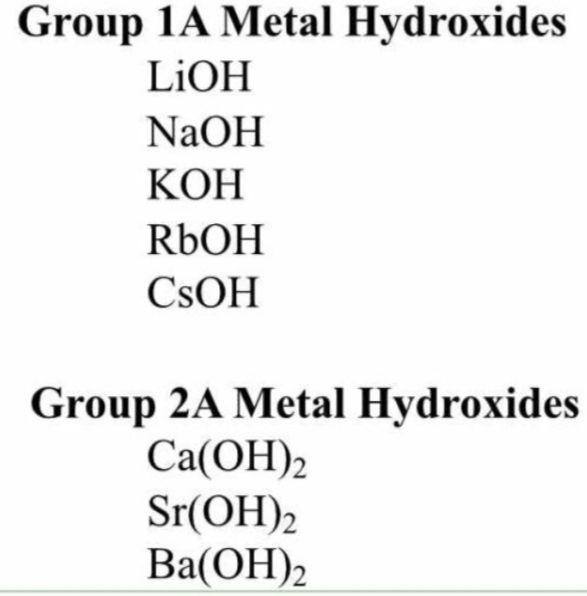
The strong bases
Easy way to remember these strong bases are the fact they're all in Group 1 and 2 of the periodic table.
If you know the H+ (or H3O+) concentration in a solution at 25 degrees Celsius, you can calculate the OH- concentration and vice versa. How do you do this?
The units are M (Molarity)

pH Formula
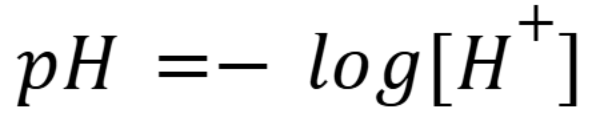
pOH formula
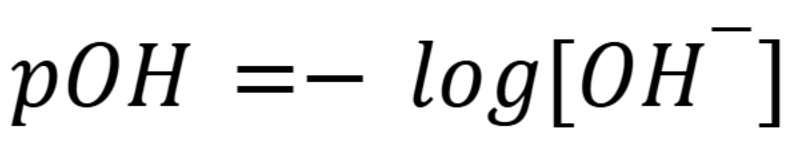
pH and pOH formula
pH + pOH = 14
Finish Unit 8
Entropy
Denoted by S. The quantity of possible energy states of the components of a system. The amount of disorder or chaos in a system. Units are Joules/Kelvin