P2.1.1 States of matter
0.0(0)
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
1
New cards
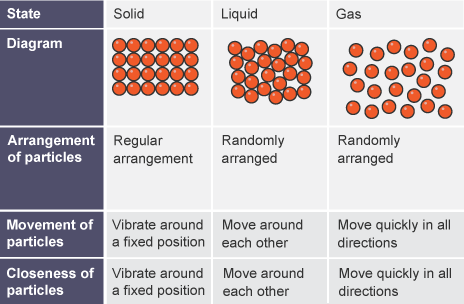
State the distinguishing properties of solids, liquids and gases
Solids: fixed shape, fixed volume, strong intermolecular forces, particles are very close together, vibrate around a fixed position
Liquids: changes shape to fit container, fixed volume, weaker intermolecular forces than solids, particles are not as packed in solids but close together, move around each other randomly
Gases: no fixed shape, changes volume to fix container, weakest attractive forces, particles far apart and move quickly
2
New cards
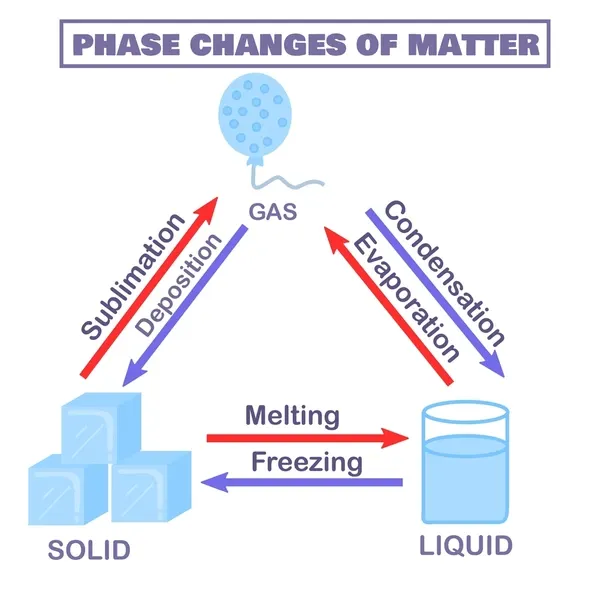
Know the terms for the changes in state between solids, liquids and gases
Solid to liquid: freezing
Liquid to solid: melting
Liquid to gas: evaporation
Gas to liquid: condensation
3
New cards
.
4
New cards
.