Lecture 2: Inflammation
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
5 steps of Acute Inflammation
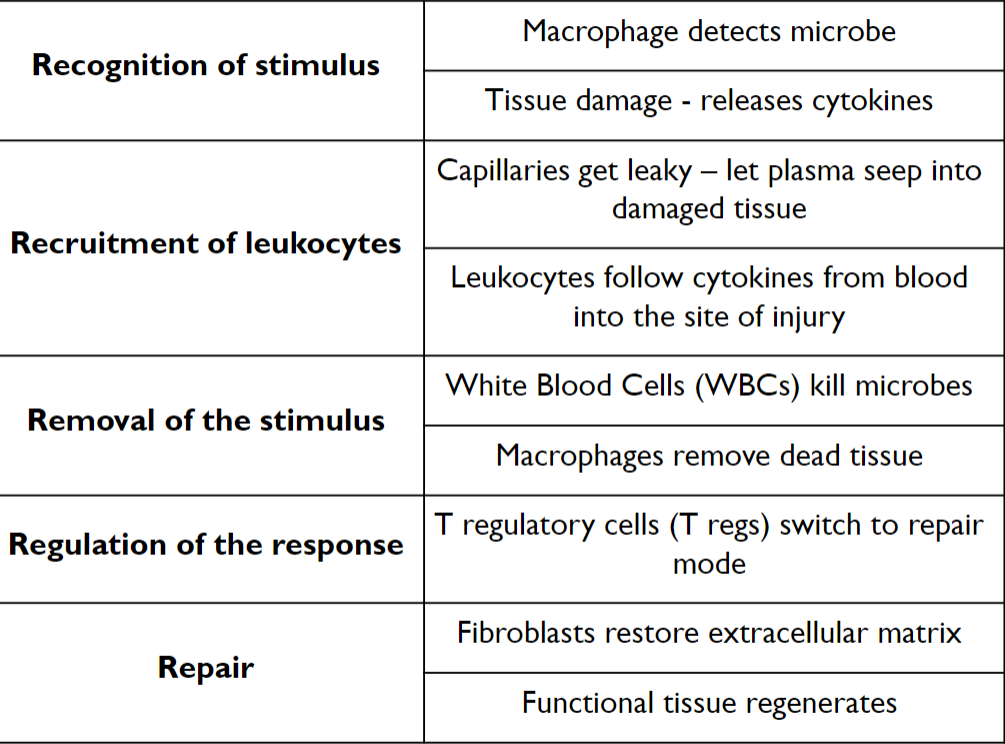
Step 1: Recognition
Cellular receptors for microbes
Toll like receptors (TLR)
Expressed on epithelial cells, macrophages, dendritic cells, lymphocytes
Sensors of cell damage
Respond to disruption of cellular homeostasis and activate production of IL-1
IL-1 activates the inflammatory cascade
DAMPS and PAMPS
Damage Associated Molecular Pattern (DAMP):
Molecules associated with cellular damage that trigger inflammatory response (heat shock proteins, mitochondrial DNA)
Often lead to production of IL-1 via inflammasomes
Pathogen Associated Molecular Pattern (PAMP):
Highly conserved pathogen molecular structure recognized by the immune system (lipopolysaccharide, some bacterial toxins)
Step 2: Recruitments of Leukocytes and Plasma Proteins
Vascular Changes
Changes in vascular flow
Increased vascular permeability
Lymphatic flow increases to drain extracellular fluid, cells and debris
Leukocyte recruitment
Leukocytes migrate through epithelium from blood into damaged region
Leukocytes follow chemokines to site of injury (chemotaxis)
Vascular Changes
Decreased flow velocity
Histamine causes vasodilation
Increased diameter reduces flow speed allowing leukocytes to slow down and stick to endothelial walls
Increased permeability (leaky capillaries)
Histamine causes endothelial contraction (gaps between cells)
Allows fluid, proteins, and cells to enter the extravascular space
Increased lymphatic flow to drain extracellular space
Leukocyte Recruitment
Flow characteristics allow leukocytes to accumulate at the endothelial cell surface moving at very low speed
Selectins allow for weak reversible attraction that produces a rolling motion
Integrins allow for a stronger bond that stops leukocytes and facilitates migration through the endothelium
Endothelial Selectin and Integrin ligand expression is promoted by cytokines (IL1, TNF)
Integrins are activated (higher affinity) by chemokines on the epithelial cell surface
Transmigration between adjacent endothelial cells is facilitated by PECAM1 (CD31) on the endothelial cells and the leukocytes
Chemotaxis
Locomotion along a chemical gradient
Injured cells and activated immune cells release chemokines
Leukocytes move toward high gradients of chemokines to the site of injury
Step 3: Removal of Stimulus
Phagocytosis and clearance of the offending agent
Neutrophils and Macrophages are the major phagocytic cells
Recognition by Phagocytic receptors
May detect microbial cell wall elements or opsonized microbes (IgG, C3b)
Engulfment of microbe
Endocytosis
Killing of infectious agent
Fusion of endosome with lysosome
May include release of granule content extracellularly (degranulation)
Neutrophils
Rapid, short lives response
Major response: degranulation
Macrophages
Slower response
Cytokine production is major functional activity
Lysosome Enzymes
Neutrophils and macrophages contain lysosomal granules with enzymes for microbial killing and tissue damage
Neutrophils
Specific (secondary) granules and Azurophil (primary) granule
Different contents but can both fuse with endosomes or release extracellularly
Neutral proteases digest host proteins like collagen, basement membrane, fibrin, elastin
Cause tissue destruction associated with acute inflammation
Neutrophil Extracellular Traps (NETS)
Fibrillar networks produced by neutrophils
Concentrate antimicrobial substances at infection sites
Trap microbes to prevent their spread
Meshwork of nuclear chromatin that binds granule proteins (antimicrobial peptide and enzymes)
Step 4: Regulation of Inflammatory Response
Anti-inflammatory mediators from macrophages and other cells: TGF beta, IL-10
Removal of stimuli via step 3
Neutrophils are self limited
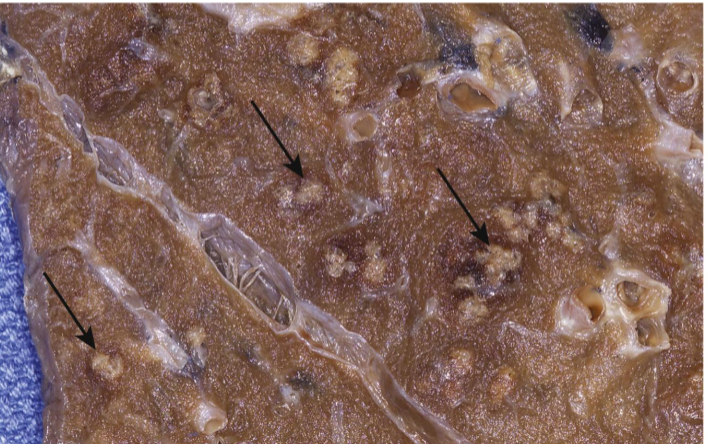
Purulent (Suppurative) Inflammation
Pulmonary abscesses – contain neutrophils and cellular debris
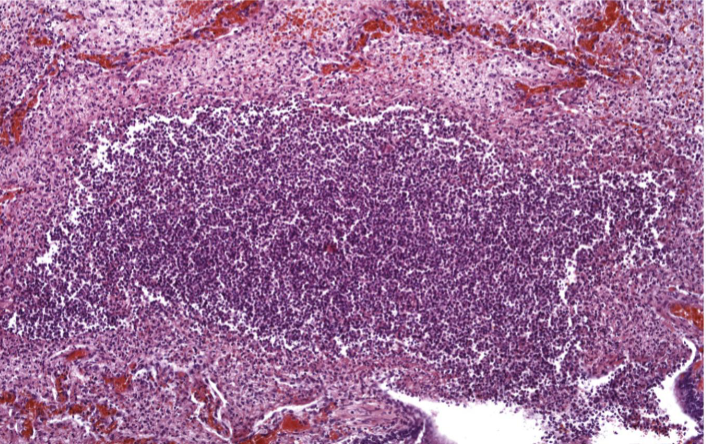
Purulent (Suppurative) Inflammation
Purulent (Suppurative) Inflammation
Acute inflammation dominated by pus – a thick exudate containing neutrophils, necrotic debris, and edema fluid
Pyogenic bacteria stimulate intense neutrophil recruitment
Liquefactive necrosis of tissue → pus formation
Gross: Yellow-white creamy fluid, localized or diffuse
Microscopic: Dense neutrophil infiltrate, tissue necrosis, protein-rich fluid
Abscess – localized collection of pus in a cavity (may have fibrous capsule if chronic)
Cellulitis – diffuse spread of purulent inflammation in tissue
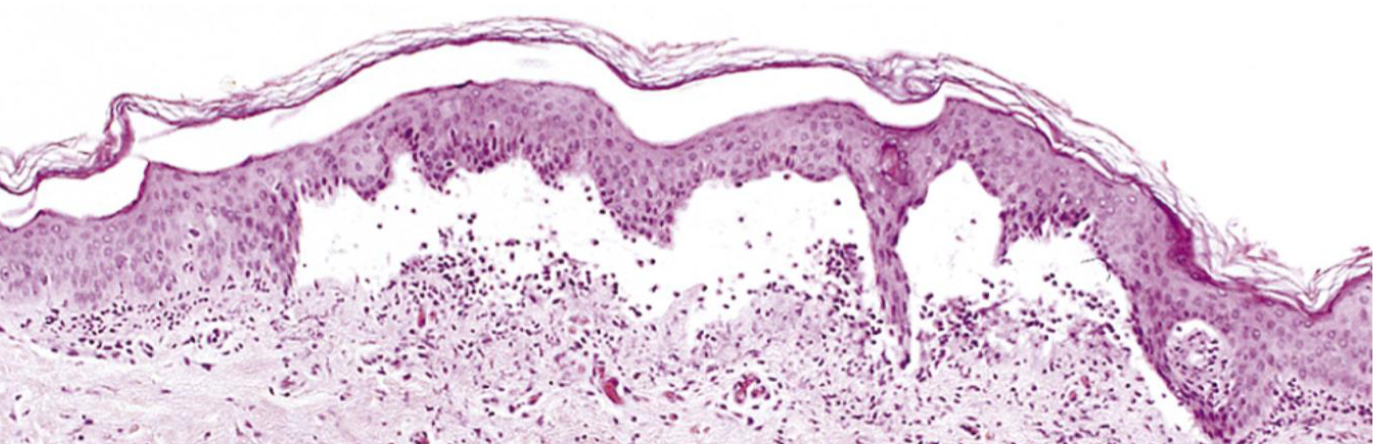
Serous inflammation
Blistered skin following thermal burn
Serous inflammation
Acute inflammation with cell-poor, protein-poor fluid (serous exudate) in a body cavity or tissue space
Mild injury to vasculature → increased vascular permeability
Plasma or serous fluid leaks into confined space
Gross: Clear or pale yellow fluid accumulation
Microscopic: Scant inflammatory cells, few proteins, mostly transudate-like fluid (but inflammatory in origin)
Skin: Burns, viral infections → blister formation
Serosal cavities: Pleura, pericardium, peritoneum (e.g., early inflammation, autoimmune disease)
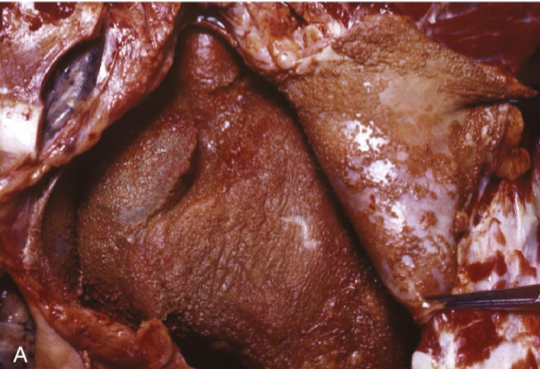
Fibrinous pericarditis (inflammation)
Deposits of fibrin on the pericardium
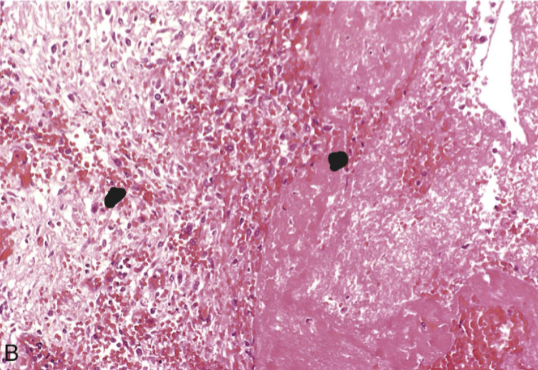
Fibrinous pericarditis (inflammation)
A pink meshwork of fibrin exudate (F) overlies the pericardial surface (P)
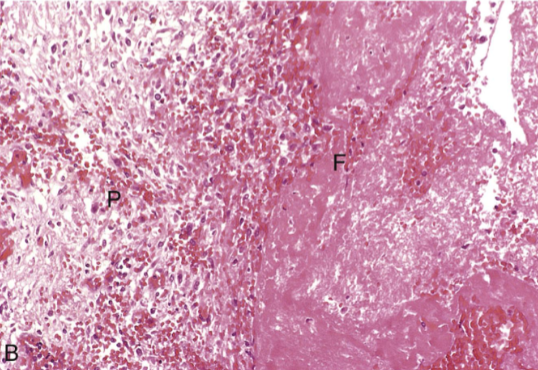
Fibrinous inflammation
Acute inflammation with fibrin-rich exudate on serosal or mucosal surfaces due to severe vascular injury
Marked endothelial damage → large plasma proteins (including fibrinogen) leak into tissue
Fibrinogen polymerizes into fibrin in the extracellular space
Gross: “Bread-and-butter” appearance — shaggy strands of fibrin between inflamed surfaces
Microscopic: Eosinophilic fibrin mesh overlying tissue; may contain inflammatory cells
Pericardium – post-MI fibrinous pericarditis
Pleura – pneumonia, uremia
Peritoneum – post-surgical inflammation
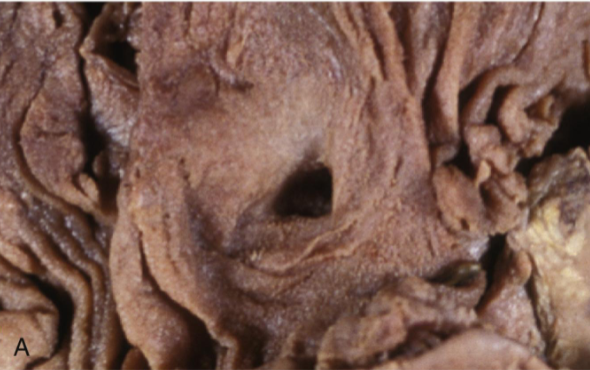
Ulcer
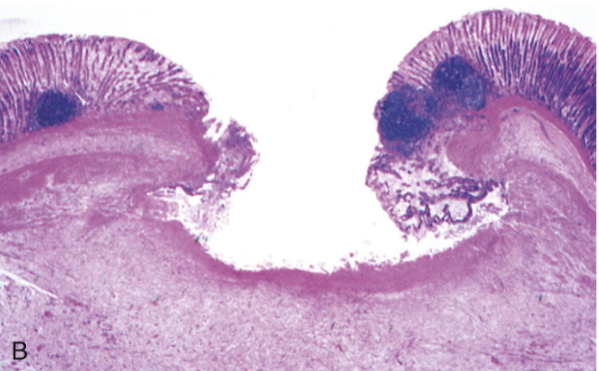
Ulcer
Ulcer
Local defect in the epithelial surface caused by sloughing of inflamed necrotic tissue
Persistent or severe injury → necrosis of surface epithelium
Acute inflammation (neutrophils) often overlies chronic inflammation (macrophages, lymphocytes)
Morphology:
Gross: Depressed lesion with irregular borders; base may contain granulation tissue or fibrinopurulent exudate
Microscopic: Necrotic debris on surface; fibrin layer; underlying inflammatory infiltrate
GI tract – peptic ulcer disease, inflammatory bowel disease
Skin – ischemic ulcers, pressure sores
GU tract – chronic infections, malignancy
Ulceration =
1) Loss of epithelium
2) Exposure of underlying connective tissue
Histamine
Source: Mast cells, basophils, platelets
Action: Vasodilation, Increased vascular permeability, endothelial activation
Prostaglandins
Source: Mast cells, leukocytes
Action: Vasodilation, pain, fever
Leukotrienes
Source: Mast cells, leukocytes
Action: Increased vascular permeability, chemotaxis, leukocyte adhesion and activation
Cytokines (TNF, IL-1, IL-6)
Source: Macrophages, endothelial cells, mast cells
Local: endothelial activation (expression of adhesion molecules)
Systemic: fever, metabolic abnormalities, hypotension (shock)
Complement
Source: Plasma (produced in liver)
Action: Leukocyte chemotaxis and activation, direct target killing (membrane attack complex), vasodilation (mast cell stimulation)
Arachidonic Acid
Precursor to many inflammatory mediators
Metabolism pathway is the target of multiple drugs
PRINCIPAL ACTIONS OF ARACHIDONIC ACID METABOLITES
Vasodilation: Prostaglandins PGI2 (prostacyclin), PGE1, PGE2, PGD2
Vasoconstriction: Thromboxane A2, leukotrienes C4, D4, E4
Increased vascular permeability: Leukotrienes C4, D4, E4
Chemotaxis, leukocyte adhesion: Leukotrienes B4, HETE
Systemic Response to Inflammation
Cytokines enter the circulation and cause a systemic response
Fever
TNF, IL-1 act on hypothalamus to raise temp
Acute-phase proteins – markers of inflammation
Produced in liver to help with immune response
C-reactive protein, SAA (serum amyloid A) bind to microbes and opsonize
Fibrinogen increases and can cause red cell adhesion
High serum ferritin (binds free iron, depriving microbes)
Leukocytosis: Colony Stimulating Factors (CSFs) stimulate production of leukocytes from precursors in the bone marrow
Neutrophils in bacterial infection, lymphocytes in viral infection
Complement System
Group of over 20 plasma proteins (including C1-C9)
Play a crucial role in host defense against microbes and inflammation
Functions in both innate and adaptive immunity
Activation leads to:
Production of cleavage products that cause increased vascular permeability, chemotaxis, and opsonization
Direct cytotoxic effect due to formation of membrane attack complex (MAC)
Vasodilation
Histamine
Prostaglandins
Increased vascular permeability
Histamine
Chemotaxis, leukocyte recruitment, and activation
TNF, IL-1
Fever
IL-1, TNF
Prostaglandins
Pain
Prostaglandins
Substance P
Acute Inflammation
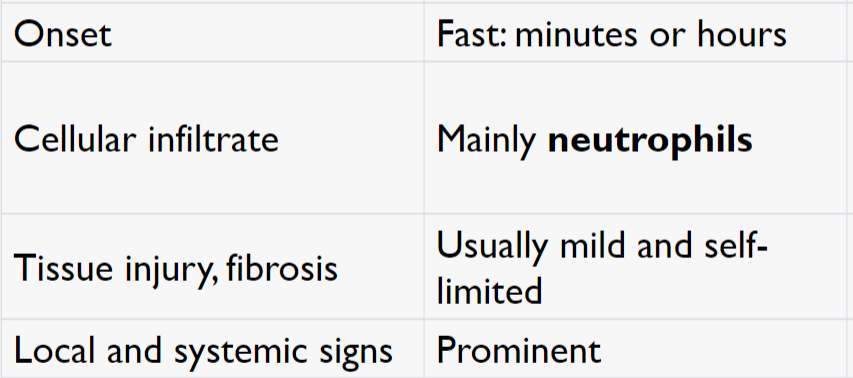
Chronic Inflammation
Onset: Slow; days
Cellular infiltrate: Monocytes/macrophages and lymphocytes
Tissue injury, fibrosis: Often severe and progressive
Less systemic and local signs
Angiogenesis (new blood vessels) and fibrosis - Healing
Acute inflammation mediators
TNF, IL-1, IL-6, Chemokines, IL-17
Chronic inflammation mediators
IL-12, IFN-y, IL-17
Chronic Inflammation Cells and Mediators
Macrophages
Lymphocytes
T Cells (CD8+ T cells – cytotoxic T cells; CD4+ T cells)
Th1 - IFN-γ - which activates macrophages (classical pathway)
Th2 - IL-4, IL-5, and IL-13:
Recruit and activate eosinophils
Alternative pathway of macrophage activation
Th17 - IL-17 - induce the secretion of chemokines
B Cells→ activated B cell → plasma cell
Source of macrophages
Activated macrophages – bone marrow (monocytes)
Resident tissue macrophages – Yolk Sac and fetal liver
Macrophage-Lymphocyte Interaction
Activated T cells produce cytokines that Recruit macrophages (TNF, IL-17, chemokines)
Activate macrophages (IFN-γ)
Activated macrophages stimulate T cells by Presenting antigens
Cytokines (IL-12)
Granulomatous Inflammation
Chronic inflammation characterized by focal aggregates of activated macrophages (epithelioid cells), often with lymphocytes and sometimes multinucleated giant cells
Cellular attempt to contain and isolate an offending agent that is difficult or impossible to eradicate
Gross: Firm, nodular lesions (may be caseating or non-caseating)
Microscopic: Central area may have necrosis (caseating) or remain viable (non-caseating); Rim of epithelioid histiocytes, multinucleated giant cells, lymphocytes
Foreign Body Granulomas – Inert material, minimal T-cell response (e.g., sutures, talc)
Immune Granulomas – Persistent T-cell–mediated immune response (e.g., TB, sarcoidosis, certain fungi).
Common Causes: Mycobacteria (e.g., Mycobacterium tuberculosis); Fungi; Foreign bodies, chronic irritants; Immune-mediated diseases (sarcoidosis, Crohn disease)
Foreign Body Granuloma
Macrophages engulfing foreign body
Commonly seen with sutures or biopsy site
Necrotizing Granuloma
Rim of epithelioid histiocytes
Central necrotic region
Multinucleated giant cells
Rim of lymphocytes and plasma cells
Non-caseating Granuloma
Well-formed granuloma = sarcoidosis
No central necrosis
Caused by T cell activation of macrophages