IHS Exam 2
1/140
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
141 Terms
_______ of pathogen and ___________ of the host affect the disease outcome
- dose
- immune status
**so just being exposed to an infectious agent (even if exposed by the appropriate route) will not necessarily cause disease (or the same level of disease) as that seen:
- in someone receiving a different level of exposure
- in someone who mounts a different level of host response to the infectious agent (innate or adaptive immunity)
Iceberg Concept of Infectious Diseases
- Infection and disease after exposure is determined by
- route of transmission
- length of exposure
- dose of inoculum
- route of acquisition
- type of infectious agent
- level of pre-existing immunity (innate or adaptive)
Damage-framework hypothesis
- microbial pathogenesis is an outcome of an interaction b/w a specific host and a specific microorganism
-pathogen dose and host immune status make a significant difference in the disease outcome
- the host-relevant outcome of the host-microorganism interaction is determined by the amount of damage to the host
- host damage can result from microbial factors and/or the host response
Function of the immune system
to detect and limit tissue damage NOT destroy microbes
- common form: tissue damage due to microorganism presence in a tissue
- this shows that the immune system is very sensitive in detecting microorganism presence in a host's tissue
How is our body home to trillions of microbes that contribute to our metabolic and immunologic health ("microbiome")?
- simple presence of a microbe is not enough to fully activate the immune system, so tissue damage is necessary
- microbes that do not cause tissue damage are tolerated (reason for gut microbiome)
Innate immunity
- "pre-set" recognition and activation mechanisms
- germ-line host-encoded
- fast
Innate and Adaptive Immunity...
work together in generating and regulating an immune response
Three core components of the immune system (innate and adaptive)
- cells
- receptors
- signal molecules
IL
- "interleukin"
- type of cytokines
Chemokines
- some chemokines are denoted CCL#, CXCL#, CX3CL#
- they bind to receptors denoted CCR#, CXCR#, CX3CR#
- type of cytokine
Other cytokines and receptors are denoted with:
various abbreviations (e.g., TNF/TNFa, IFNg, etc.)
CD
- "cluster of differentiation" (ex: CD47, CD4, etc)
- cell surface molecules
What cells are most important for the immune system?
- white blood cells (leukocytes)
- found in every tissue in the body during health AND disease
Leukocytes are derived from:
the bone marrow via hematopoiesis thru replication and differentiation
Leukocytes circulate:
in the blood
**a subset of lymphocytes (specific type of WBC , T & B cells) ALSO circulate via lymphatics
Order of Circulatory System:
Arteries -> Capillaries -> Veins -> Lungs -> Heart -> Arteries -> etc.
(ACVLH) - Ants Climb Very Large Hills
As a part of the circulatory system, leukocytes leave the circulation and...
move into tissues at capillary beds
What is so important about capillaries?
- all tissues have capillaries
- oxygen, nutrient, metabolite, and toxin exchanges all occur in capillary beds
- fluid movement INTO tissues also happens via capillaries
fluid movement OUT OF tissues largely happens via
lymphatic vessel
Where are leukocytes found? Especially since they are "found in every tissue during health and disease"?
- in the tissue itself (resident, recruited from blood vessels)
- in specialized structures (MALT)
- in lymph nodes associated with the tissue
What are lymph nodes?
- specialized, encapsulated tissue in the lymphatic system that contains lymphocytes and myeloid mononuclear cells
- lymph is filtered thru lymph nodes
Lymph
- a fluid that contains white blood cells and bathes bodily tissue
- similar in content to serum/plasma
- interstitial fluid
Lymph is derived from:
blood, digestion, and tissue metabolism
Lymph flows thru _________
Lymph is collected by _________
Lymps is dumped __________
- flows throughout tissue
- collected by lymphatic vessels (along with WBC)
- dumped into circulation @ the thoracic duct
lymph vessels involve:
endothelial cells
Lymphatic System
an open-ended second circulatory system in the body
connects the tissue sites to the lymph nodes, spleen, and circulatory system
Where are capillary beds found?
in ALL vascularized tissue ("blood vessel containing") in the body
Hematopoiesis
the production of blood cells
HSC
Hematopoietic Stem Cells (HSCs) in bone marrow differentiate into Myeloid Stem Cells and Lymphoid Stem cells
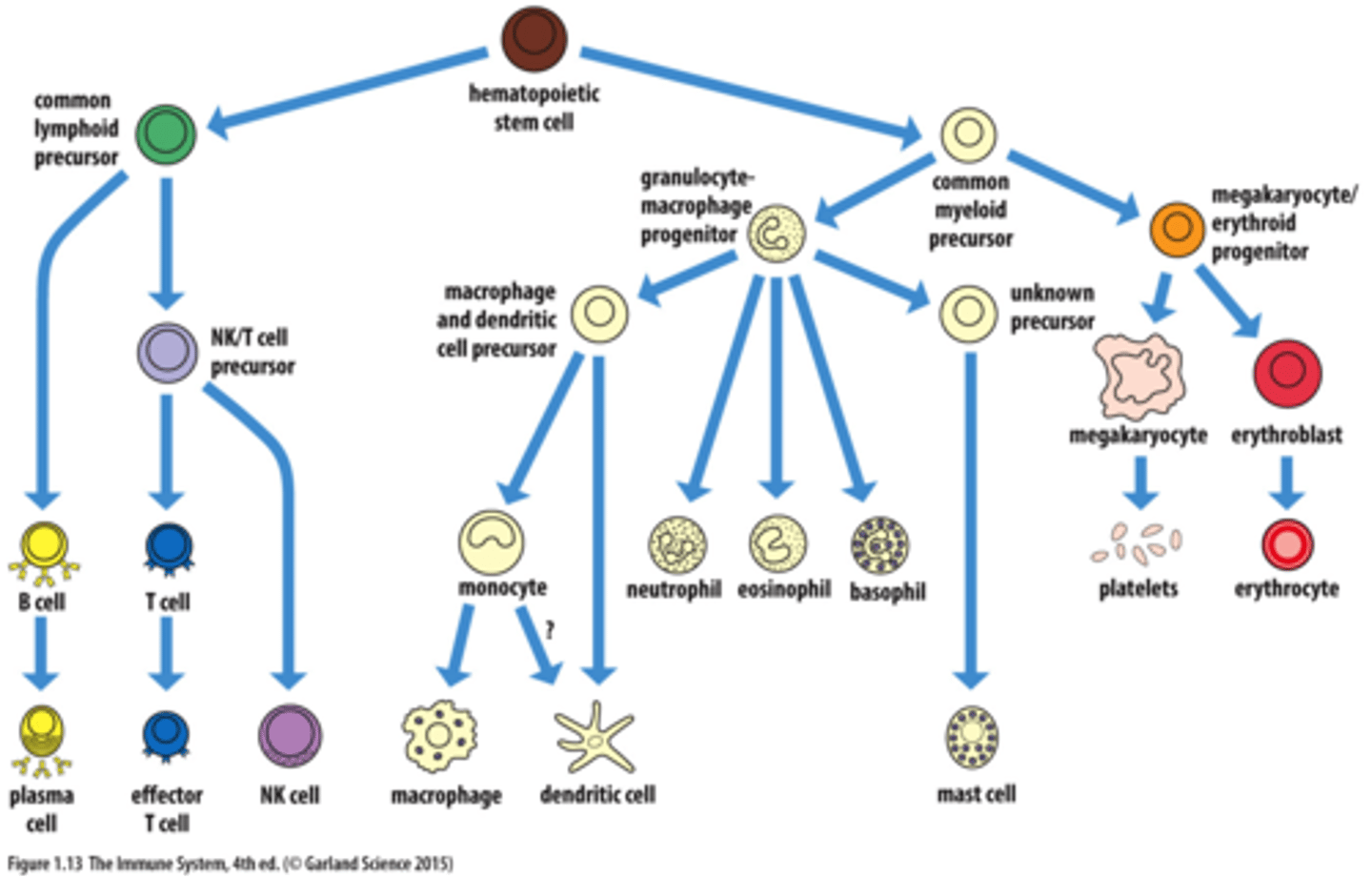
Myeloid Lineage of Hematopoiesis (bone marrow)
Myeloid stem cells differentiate into Mast cells, Myeloblasts, and Monoblasts
- Myeloblast --> eosinophil, basophil, neutrophil
- Monoblasts --> Monocyte --> Macrophage, Dendritic Cells
Lymphoid Lineage of Hematopoiesis
Lymphoid stem cells differentiate into Natural Killer Cells, T cells, B cells, and Innate Lymphoid Cells
- T cells processed in the Thymus
- B cells processed in the Bone marrow
Structure of blood vessels
all composed of 3 main cellular layers (except capillaries):
inner--> outer
- tunica intima (endothelium), tunica media, and tunica adventitia
capillaries have endothelial and pericyte?
Major cell types that compose blood vessel layers
- endothelial cells make the intima (think "endo"/"in)
- vascular smooth muscle cells make the media
- fibroblasts make the adventitia
Pericytes
a specialized type of fibroblast
5 cardinal signs of inflammation
- redness
- warmth
- pain
- swelling
- altered function
Inflammation is characterized by
5 cardinal signs of inflammation (for external tissue of joints)
OR
- leukocyte infiltrates into a tissue
- increased vascular permeability
- enhanced cytokine production in tissue
Leukocytes can either be:
- resident in tissue
- recruited into tissue from blood during inflammation
General Steps of Infection & Inflammation
1) Infection via splinter
2) Resident macrophages engulf pathogens and release cytokines (like alarm signals)
3) Vasoactive factors and cytokines help deliver additional phagocytes
- we also se extravasating ("leaking") phagocytes
4) Some cytokines initiate healing as pathogens are destroyed
monocyte-derived dendritic cells
are the most numerically dominant in the body, but there are other types of DC, also
polymorphonuclear leukocytes (PMNs)
eosinophil, basophil, neutrophils
- contain granules that are released during infection
Receptors (core component of immune system)
receptor-ligand interactions are the primary molecular mechanism that drives the actions and functions of the immune system
What happens after a receptor binds its ligand?
- the basic concept is that only one ligand binds a specific receptor, however, there are exceptions and modifications to this simple rule
_______________
1) binding of the extracellular ligand to receptor causes a change in the intracellular portion of the receptor
2) this leads to the generation of "second messenger" molecules in the cytosol
3) secondary messengers alter specific aspects of transcription and/or translation leading to a change in the biological activity of the cell
Degenerate receptors (exception to one ligand/one specific receptor concept)
- receptors that bind multiple structurally similar ligands OR receptors that bind multiple ligands that exhibit similar surface charge
- the affinity of degenerate receptors for their various ligands will likely be different and may affect the generation of secondary messengers
Cross-reactive ligands (exception to one ligand/one specific receptor concept)
- ligands whose receptor binding site on the ligands are structurally identical but differ in the molecular structure outside the binding site for the receptor
- ligands whose receptor binding site is somewhat similar to the primary ligand of that receptor
(this receptor-ligand interaction is analogous to that of a degenerate receptor interaction)
Degenerate receptors vs. cross-reactive ligands
- Specific receptors function in signaling networks as a result of their “lock & key” specificity” (so basically a ligand binds a receptor and generates a signal)
HOWEVER, exceptions:
- degenerate receptors function by having broad specificity (still have a strong degree of specificity)
- cross-reactive ligands are usually "unintended" with non-primary ligands having "less than optimal" cellular responses b/c optimal responses happened w/ the primary ligand
Major ligands for the immune system receptors
- antigens
- microbe-associated molecular patterns (MAMPs)
- damage-associated molecular patterns (DAMPs)
- growth factors and cytokines
- hormones
- bioactive lipids
- others...
Antigen (Ag) are recognized by:
- recognized by the antigen receptors of the adaptive immune system: antibodies and T cell receptors (TCR)
Antigens are usually:
- usually a protein but can be a carbohydrate (or rarely DNA or lipid)
Antigens are derived from:
can be derived from microorganisms (eg. bacteria, virus, parasite), environmental particle, food, drug, another creature, etc
Some other types of antigens
allergens and autoantigens
Allergen (type of Ag)
an antigen derived from a non-infectious source
Autoantigen (type of Ag)
a host-derived molecular that the immune system targets in autoimmunity
Microbe-associated molecular patterns (MAMPs)/PAMPS ("pathogen...")
- small molecular structures that are conserved within a class of microorganisms and recognized by degenerate receptors of the innate immune system b/c broad/pattern
eg. unmethylated CpG in bacterial DNA (humans have methylated)
eg. mannans in yeast cell walls
Pattern recognition receptors (PRRs)
host-encoded degenerate receptors that bind to MAMPs, thus "identifying" microbe presence in tissue
- expressed on phagocytic white blood cells and many other cells in the body
- found on both outside and inside cell!!
Damage associated molecular proteins (DAMPs)
- derived from host cell including tumor cells, dead or dying cells, or products released from cells in response to signals such as lack of oxygen
- introduce what's known as "sterile" inflammatory responses because they are derived from host cellular contents
- created in environments of trauma, ischemia (lack of blood flow), or tissue damage
- THEY DO NOT REQUIRE INFECTION!
- diff PRRs can also bind to diff DAMPs
Antibody
- a heterodimeric bivalent receptor specific for an antigen
- heterodimeric b/c made up of 4 polypeptides: 2 “heavy chains” and 2 “light chains” joined by disulfide bonds to form a "Y" shaped molecule
- can bind two identical Ag molecules
Immunoglobulin/antibody
B lymphocytes aka B cells produce antibodies (they are the only source)
Surface Ig
- Membrane-Bound Ig
- Found on B cell surfaces as part of the B cell receptor (BCR) complex
Antibody structure
protein made up of 4 polypeptides: 2 "heavy chains" and 2 "light chains" joined by disulfide bonds to form a "Y" shaped molecule
Antibody-antigen binding
antibody binds a specific antigen at its antigen-binding site with a lock-and-key interaction (receptor-ligand interaction)
**Here, Ag is the molecule recognized by the receptors of the immune system (eg. antibodies)
Epitope
the specific molecular structure on an antigen that is specifically bound by antigen receptors on Ab (can approach almost covalent interactions)
Antigen receptors
immunoglobulin, T cell receptor
adaptive immunity
- "flexible" recognition and activation mechanisms
- generated through somatic cell DNA rearrangement and mutation
- slower (but can speed up upon repeated exposure to the same insult)
Genomic rearrangements to generate an antibody
- random rearrangement of V, D, J in heavy chain
- random rearrangement of V, J in light chain
*a single B cell randomly selects which light chain gene locus to use and others are silenced for the life of that cell and all of its subsequent clones
Key Stages of B cell development
1) Stem Cell → The process begins with a hematopoietic stem cell (HSC)
2) Pro-B Cell → Starts VDJ recombination. DJ FIRST FOR HEAVY
3) Pre-B Cell → Completes heavy chain recombination (V-DJ) and expresses heavy chains with surrogate light chains.
3) Immature B Cell → Expresses IgM on the membrane and completes light chain rearrangement (kappa or lambda light chain)
4) Mature B Cell → Expresses both IgM and IgD on its surface.
5 +6) Mature B cells can differentiate into Memory B cells which have undergone activation These Memory B cells can further differentiate into Plasma cells
5) Mature B cells can differentiate into Plasma Cell → A fully differentiated B cell that secretes antibodies (IgM initially or class-switched IgG, IgA, or IgE).
Bone Marrow (Antigen-INDEPENDENT)
Early B cell development (from stem cell to mature B cell) occurs in the bone marrow. This phase involves gene rearrangement
Periphery (Antigen-DEPENDENT)
Once mature B cells enter the bloodstream and secondary lymphoid organs (e.g., spleen, lymph nodes, mucosa-associated lymphoid tissue MALT), they encounter antigens and undergo::
- Activation
- Class switching (switching from IgM to IgG, IgA, or IgE)
- Differentiation into memory B cells or plasma cells
- B cell activation occurs in the peripheral lymphoid organs, so B cells are activated when they are in their mature state, not immature, meaning they have migrated from the bone marrow to secondary lymphoid tissues and express both IgM and IgD.
- B cell activation requires antigen binding to the B cell receptor (BCR)
Innate vs. Adaptive Receptors
- Innate immunity uses pattern recognition receptors (PRRs) to recognize conserved pathogen-associated molecular patterns (PAMPs)
- while adaptive immunity employs antigen-specific receptors on T and B cells, generated through gene rearrangements, to recognize a vast diversity of antigen
Signal molecules
critical for cell-to-cell communication in the body and regulation of physiological response
- eg. growth factors, cytokines, hormones, bioactive lipids
Cytokine
- protein that binds to a specific receptor
- intercellular signaling molecule
can act locally or systemically
- can have multiple effects, depending on the target cell binding the cytokine
What is responsible for a person feeling terrible during infection or in response to a strong vaccine?
cytokines
endothelium
lines the inner surface of all blood vessels
epithelium
Line external surfaces of the body, such as the skin, mucous membranes (e.g., respiratory and digestive tracts), and internal cavities (e.g., lungs, bladder).
Tissue Macrophages (the Reticuloendothelial System)
Macrophages are found throughout the body in all tissues and many are derived from monocytes that have left the blood
- monocytes are derived from monoblast
*Blood monocytes can become an "inflammatory" macrophage at any infection site
Neutrophils
- most abundant white blood cell in the body
- derived from myeloblast
- granulocyte (type of PMN leukocyte)
- "ambulance" of the immune system: a lot is produced quickly in bone marrow upon infection and they travel to the infection site to exert anti-microbial activity
- typically the predominant cell in pus
Other name for neutrophils
polymorphonuclear leukocytes
due to the appearance of their nucleus
Phagocytosis
when a cell uses its plasma membrane to engulf a large particle, giving rise to a phagosome (internal compartment)
- type of endocytosis
- major mechanism used to remove pathogens and cell debris
What cells primarily carry out phagocytosis
macrophages (mostly derived from monocyte) and neutrophils (derived from myeloblasts)
For phagocytosis to proceed...
phagocytes must first recognize the surface of a particle as foreign
Phagocytosis receptors
- a receptor-mediated process
- enhanced by opsonization
Opsonization
opsonins (eg. Ab, complement C3b, or other opsonins) coat pathogens, making them more easily recognized and engulfed by macrophages for destruction.
- all opsonins bind to a specific receptor on a phagocyt
Antibody-mediated Opsonization example
1) extracellular bacteria apprach macrophage
2) Opsonization: Ab binds to bacteria and the Fc region of Ab binds to macrophage Fc receptors
3) Ingestion by macrophage
Opsonin-independent "Scavenger" receptors
- important in clearing particulates, old erythrocytes, and apoptotic cells
CD47
- cell-surface glycoprotein CD47
- expression of CD47 by a host cell signals to phagocytes to prevent phagocytosis of that cell even when bound by an opsonin or via opsonin-independent pathways (“don’t eat me” signal)
Macrophages have:
phagocytic receptors that bind microbes and their complements
Steps in phagocytosis
1) Adherence → The bacterium binds to the surface of a phagocytic cell. Antibodies or complement proteins (opsonins) help in binding.
2) Engulfment → The phagocyte extends pseudopods around the bacterium and engulfs it.
3) Phagosome Formation → The bacterium is enclosed within a phagosome inside the phagocyte.
4) Phagolysosome Formation → The phagosome fuses with a lysosome, forming a phagolysosome.
5) Microbe Destruction → Enzymes and reactive oxygen species (ROS) from the lysosome break down the bacterium.
6) Debris Release → The degraded microbial debris is expelled from the cell.
Killing ingested microbes
- phagocytic cells use reactive oxygen radicals to kill ingested bacterial cells by oxidizing key cellular constituents of the ingested microbe
- "respiratory burst"
- occurs within the phagocytic vacuole of the phagocyte, which is not damaged by the toxic oxygen products
- degradative enzymes are also found in the phagolysosome
Respiratory burst
a series of catalyzed reactions in the phagolysosome that creates reactive oxygen metabolites such as peroxide, hypochlorite, nitric oxide, superoxide, hydroxyl
Acute inflammatory response cytokines
- the cytokines TNF-alpha, IFN-gamma, IL-1, and IL-4 are well-characterized stimuli that induce endothelial cell adhesion molecule expression
- the cytokine IL-6, as well as C3a, C4a, C5a and histamine, signal to endothelial cells to promote endothelium (vascular) barrier leak
- chemokines (such as IL-8/CXCL8) are a class of cytokines that signal for leukocyte migration (chemotaxis) to the site
cytokines TNF-alpha, IFN-gamma, IL-1, and IL-4
stimuli that induce endothelial cell adhesion molecule expression
cytokine IL-6, as well as C3a, C4a, C5a and histamine
signal to endothelial cells to promote endothelium (vascular) barrier leak
Chemokines (such as IL-8/CXCL8)
class of cytokines that signal for leukocyte migration (chemotaxis) to the site of infection
Migration of NEUTROPHILS into an inflamed or infected tissue
1) Rolling Adhesion: Selection-mediated adhesion:
S-Lex binds to E-selectin which slows down blood cells - allowing leukocytes to roll adhesion along the vascular endothelial surface
2) Tight Binding: interaction is strong enough that it can tightly bind specific receptors on the endothelium and stop
3 & 4) Diapedesis & Migration: Tight junctions start to loosen and neutrophils secrete proteases which helps them push through cells (squeeze by without fully opening and then reseal)
- Neutrophil chemokines include CXCL1, CXCL2, CXCL8 (IL-8)
- Neutrophil detects CXCL8 and follows increasing concentration to the site of infection
4) Migration of Moc
Migration of MONOCYTE into an inflamed or infected tissue
1) Monocyte binds adhesion molecules on vascular endothelium near infection site and receives chemokine signal
- Monocyte chemokines include CCL2, CCL3, CCL4, CCL5, CX3CL1
2) Monocyte migrates into surrounding tissue
3) Monocyte differentiates into inflammatory monocyte at infection site
Innate Immunity, where tissue resident cells:
detect cellular/tissue damage and microorganisms … and in response, induce an inflammatory response to contain the infection by producing cytokines and other mediators
Inflammatory response and blood clotting
Microbial recognition and tissue damage initiate an inflammatory response via cytokines, including chemokines
1) cytokines produced by macrophages cause dilation of local small blood vessels
2) leukocytes move to periphery of blood vessel due to increased expression of adhesion molecules by endothelium
3) leukocytes extravasate (leak) at infection site
4) blood clotting in microvessels
cytokine (internet def)
Certain cells release cytokines while other cells contain cytokine receptors. Think of a cytokine as a key and the receptor on the receiving cell like a lock.
When the cytokine (key) enters the cytokine receptor (lock), the receiving cell receives a message that tells it what to do. The cell acts based on the message it receives.
For example, an immune cell may detect a harmful substance in your body, like a virus, and release cytokines in response. Cytokines can travel through your bloodstream or directly into tissue until they reach a cell with the matching receptor. Once the cytokine binds to the receptor, the receiving cell receives instructions and acts on them. For instance, the cell may travel to the virus and attack it. It may increase its defenses to prevent viruses from invading.
Toll-like receptors (TLR)
- transmembrane proteins
- some TLRs are expressed on the cell surface where they can detect extracellular MAMPs
- TLRs located intracellularly (in the walls of endosomes) can recognize MAMPs such as dsRNA, ssRNA, unmethylated CpG DNA that are only accessible after the microorganism has been broken down after phagocytosis
Cytosol vs vesicular Compartments
- The cytosol contains freely floating molecules, while vesicular compartments (e.g., endosomes, secretory vesicles) transport and process materials within the cell.
- Endocytosis creates endosomes, and phagocytosis is a specific type of endocytosis that targets large particles.
Ig Heavy Chain production
1) D to J recombination first
2) then V to DJ recombination
3) then transcription and splicing
4) then translation and assembly