Microbial Physiology 450 Exam 2
5.0(1)
Card Sorting
1/116
Earn XP
Description and Tags
Secretory systems, Gene activation/repression with ROS, etc
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
117 Terms
1
New cards
What is the function of DsbB?
It is a thiol:disulfide oxidoreductase.
* Maintenance and formation of disulfide bonds in proteins
* Maintenance and formation of disulfide bonds in proteins
2
New cards
What is the driving force of protein folding?
H2O
3
New cards
What does the GroEL/GroES complex consumes ATP for?
To unfold misfolded proteins and facilitate correct folding
4
New cards
Which one is not a peptidyl prolyl isomerase?
FimC
5
New cards
Why are peptidyl prolyl isomerases required for protein folding?
Trigger factor/ catalyzing factor
6
New cards
What is the molecular biology central dogma?
Replication
(DNA→mRNA)-Transcription
(mRNA→protein)-Translation
(DNA→mRNA)-Transcription
(mRNA→protein)-Translation
7
New cards
What is the driving force of protein folding?
Water molecules drive protein folding through burying hydrophobic patches inside
8
New cards
What are the functions of Chaperone proteins?
* assist polypeptides to self-assemble by inhibiting misfolding and undoing misfolding
1. N-terminus is first synthesized to prevent misfolding
2. Secreted proteins are bound to these proteins
3. This protein prohibits denatured proteins from forming aggregates
1. N-terminus is first synthesized to prevent misfolding
2. Secreted proteins are bound to these proteins
3. This protein prohibits denatured proteins from forming aggregates
9
New cards
What causes increased production of chaperone proteins?
Stress induced (ie. heat shock proteins increasing)
10
New cards
What happens when a protein cannot fold correctly?
1. Forms aggregates
2. Refolded by chaperone proteins
3. Degraded by ATP-dependent proteases
11
New cards
What are the major chaperone proteins in bacteria?
tig- Trigger factor
DnaK/Hsp70- Heat shock protein 70
GroEL/GroES
DnaK/Hsp70- Heat shock protein 70
GroEL/GroES
12
New cards
What is the simple folding pathway of bacteria?
Trigger factor (tig)→DnaK/DnaJ/GrpE→GroEL/GroES
13
New cards
What is used to unfold misfolded proteins and facilitate proper folding?
ATP
14
New cards
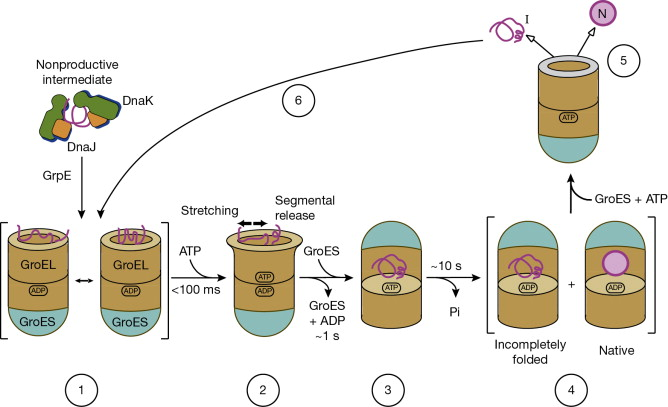
How does GroEL properly fold a protein?
* Will take in misfolded protein and produce properly folded protein
Relaxed formation accepts protein from DnaK/DnaJ→ATP and GroES tighten the conformation→ properly folded protein is produced
Relaxed formation accepts protein from DnaK/DnaJ→ATP and GroES tighten the conformation→ properly folded protein is produced
15
New cards
What are the three subclasses of Molecular chaperones?
Folding- Chaperones (**DnaK** and **GroEL**) rely on ATP-driven conformational change
Holding- Chaperones (IbpA, IbpB, Hsp31, Hsp33, t**rigger factor**, **SecB, FimC**) maintain partially folded proteins on their surface to await availability of folding chaperone
disaggregating- Chaperone (ClpB) promotes the solubilization of proteins that have become aggregated as a result of stress
Holding- Chaperones (IbpA, IbpB, Hsp31, Hsp33, t**rigger factor**, **SecB, FimC**) maintain partially folded proteins on their surface to await availability of folding chaperone
disaggregating- Chaperone (ClpB) promotes the solubilization of proteins that have become aggregated as a result of stress
16
New cards
What are the problems Periplasmic chaperones face?
No ATP, nor reducing power available
17
New cards
What are the Disulfide bond (Dsb) enzymes?
Periplasmic chaperones
* DsbA: catalyzes disulfide bond formation, is a strong oxidant
* DsbB: thiol:disulfide oxidoreductase, to keep DsbA in the oxidized form; passes electrons to CoQ of ETS
* DsbC & DsbD: Isomerases, functional only in the reduced form \\n (-SH, instead of –S-S-)
* DsbD: disulfide bond reductase (membrane located) to keep DsbC \\n and DsbG in the reduced form
* DsbA: catalyzes disulfide bond formation, is a strong oxidant
* DsbB: thiol:disulfide oxidoreductase, to keep DsbA in the oxidized form; passes electrons to CoQ of ETS
* DsbC & DsbD: Isomerases, functional only in the reduced form \\n (-SH, instead of –S-S-)
* DsbD: disulfide bond reductase (membrane located) to keep DsbC \\n and DsbG in the reduced form
18
New cards
Match the functions of the following chaperon proteins.
(Trigger Factor, DnaK, ClpB, GroEL&S, FimC, PpiD)
to
(peptidyl prolyl isomerase, peptidyl prolyl isomerase, peptidyl prolyl isomerase/holding chaperon, holding chaperon, disaggregating chaperon, folding chaperon)
(Trigger Factor, DnaK, ClpB, GroEL&S, FimC, PpiD)
to
(peptidyl prolyl isomerase, peptidyl prolyl isomerase, peptidyl prolyl isomerase/holding chaperon, holding chaperon, disaggregating chaperon, folding chaperon)
Trigger Factor- peptidyl prolyl isomerase
%%DnaK- folding chaperon%%
==ClpB- disaggregating chaperon==
GroEL^^&S^^- peptidyl prolyl isomerase/^^holding chaperon^^ %%(Folding Chaperone too)%%
^^FimC- holding chaperon^^
PpiD- peptidyl prolyl isomerase
%%DnaK- folding chaperon%%
==ClpB- disaggregating chaperon==
GroEL^^&S^^- peptidyl prolyl isomerase/^^holding chaperon^^ %%(Folding Chaperone too)%%
^^FimC- holding chaperon^^
PpiD- peptidyl prolyl isomerase
19
New cards
What is the function of DsbG?
Same as DsbC, coorrection of misfolded protein S-S bonds with their S-H bonds.
20
New cards
Why are ATP-dependent proteases important to cells?
1. Removing junk proteins
2. Regulating cell cycle
3. Regulating metabolism associated with growth phase
21
New cards
Which one is false about ATP-dependent proteases?
* They are in the cytoplasm
* They consume ATP for protein degradation
* They degrade protein substrates supplied in a growth medium
* They degrade damaged proteins
* They are in the cytoplasm
* They consume ATP for protein degradation
* They degrade protein substrates supplied in a growth medium
* They degrade damaged proteins
They degrade protein substrates supplied in a growth medium
22
New cards
What is the function of tmRNA?
1. Combines the function of tRNA and mRNA
2. Stop translation of truncated proteins stalled on damaged or truncated mRNA
3. Tag the truncated proteins for degradation by ATP-dependent proteases
23
New cards
What is the function of the adaptor protein ClpS?
Help ClpP/ClpA to degrade N-degron-containing proteins
24
New cards
What diseases are associated with misfolded proteins?
1. Due to Aggregated proteins: Mad Cow Disease, ==**Alzheimer’s disease, Huntington’s**==, Parkinson’s disease
\
2. Due to loss of function: Emphysema, ==**Cystic fibrosis**==, Lysosomal storage disease
25
New cards
What are the two groups of proteins in the cytoplasm that are subject to ATP-dependent proteases?
* Degradation of abnormal proteins (misfolded or damaged)
* short-lived regulatory proteins
* short-lived regulatory proteins
26
New cards
What are Secreted proteases (exoenzymes)?
* extracellular
* energy-independent degradation of proteins
* used for consuming protein substrates
* energy-independent degradation of proteins
* used for consuming protein substrates
27
New cards
What are Intracellular proteases?
* ATP-dependent for protein (substrate) unfolding.
(AAA+ protease)
* Intracellular
* for cellular maintenance ==(not for consuming substrates)==
\
Examples:
1. Lon protease (mutants are longer)
2. FtsH (Fts: filamentous temperature sensitive mutants)
3. Proteosomes ClpP/ClpA, ClpP/ClpX, ClpY/ClpQ
(AAA+ protease)
* Intracellular
* for cellular maintenance ==(not for consuming substrates)==
\
Examples:
1. Lon protease (mutants are longer)
2. FtsH (Fts: filamentous temperature sensitive mutants)
3. Proteosomes ClpP/ClpA, ClpP/ClpX, ClpY/ClpQ
28
New cards
What does a Lon protease do?
* target abnormal proteins & cell cycle proteins
* homohexamer (Consists of 6 identical proteins making a hexagon)
* endo protease (Breaks peptide bonds)
* homohexamer (Consists of 6 identical proteins making a hexagon)
* endo protease (Breaks peptide bonds)
29
New cards
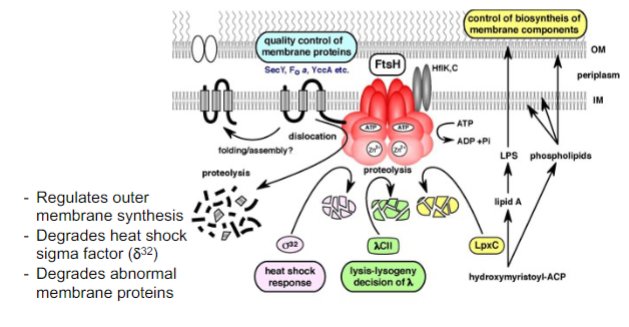
What is FtsH proiteins function?
* Regulates protein break down from environmental responses
* membrane anchored
* Zn2+protease
* AAA+domain for ATP binding and hydrolysis
* membrane anchored
* Zn2+protease
* AAA+domain for ATP binding and hydrolysis
30
New cards
What are Proteosome ClpY/ClpQ (HslU/HslV) functions?
* ClpY: Takes in unfolded substrate with ATP hydrolysis, passing the linear peptide to ClpQ-Chaperone ATPase
* ClpQ: Chamber of protein degradation-two 6 membered rings to form chamber capped with two 6-membered ClpY ring caps
* ClpQ: Chamber of protein degradation-two 6 membered rings to form chamber capped with two 6-membered ClpY ring caps
31
New cards
What are Proteosome ClpA/ClpP functions?
ClpA: Unfolds substrate with ATP hydrolysis and pass the linear peptide to ClpP
ClpP: Chamber of degradation
ClpP: Chamber of degradation
32
New cards
What is the function of Proteosome ClpX?
Acts as ATPase and replaces ClpA’s functions
* Unfolds substrate protein with ATP hydrolysis
* Unfolds substrate protein with ATP hydrolysis
33
New cards
What is the similar to ClpXP?
What is similar to ClpAP?
34
New cards
What is ClpB’s function?
disaggregating chaperone
35
New cards
What is a N-degron?
Certain amino acids at the N-terminus act as promoters for intracellular degradation (Signal for degradation)
36
New cards
What is ClpS (adaptor) function?
* Contains a N-recognition domain that is hydrophobic and negatively charged.
* Aids ClpAP in linearizing protein substrate.
* Turns off SsrA-tagged protein (signal) degradation by ClpA.
* Aids ClpAP in linearizing protein substrate.
* Turns off SsrA-tagged protein (signal) degradation by ClpA.
37
New cards
SsrA-tagged proteins (C-terminal tag) functions?
Codes for TmRNA: transfer and messenger.
Stops translation for damaged truncated proteins on ribosomes without stop codons.
Stops translation for damaged truncated proteins on ribosomes without stop codons.
38
New cards
What degrades SsrA-tagged proteins?
Degraded by ClpAP or ClpXP proteases
39
New cards
How does the ribosome release a stalled protein from a broken mRNA?
SspB protein specifically binds ssrA tag→ enhancing binding of the tagged protein to ClpX.
TLDR: SsrB calls ClpX to the protein for substrate refolding/other
TLDR: SsrB calls ClpX to the protein for substrate refolding/other
40
New cards
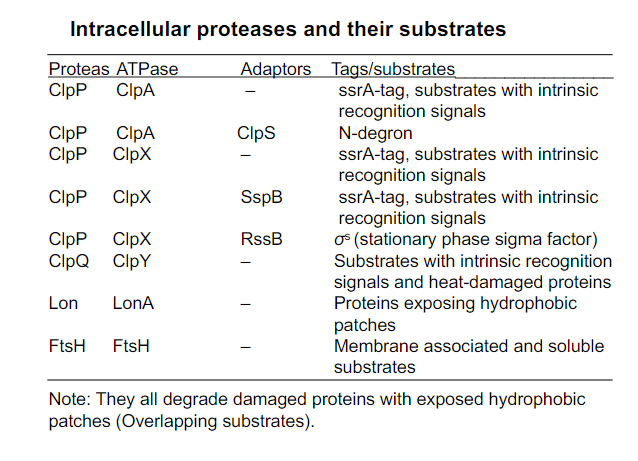
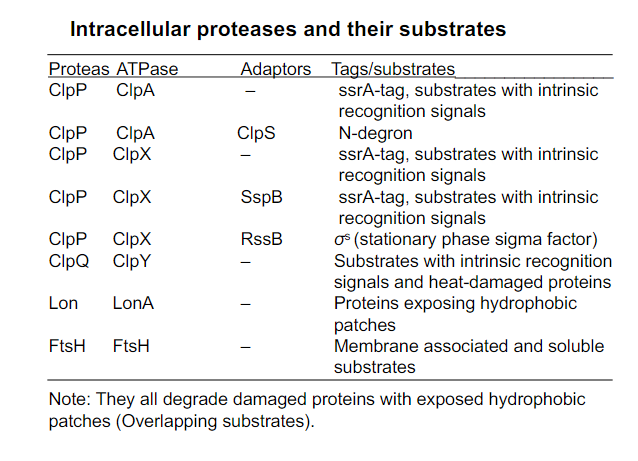
Intracellular proteases and their substrates (overall)
Focus on ClpAP, and ClpYQ
41
New cards
Why does a bacterium have several AAA+ proteases?
* Essential to activities
* Activities of each proteases are different
* Natural antibiotic targets specify AAA+ proteases
* ==__**Overlap in activates to ensure survival**__==
* Activities of each proteases are different
* Natural antibiotic targets specify AAA+ proteases
* ==__**Overlap in activates to ensure survival**__==
42
New cards
Loss of function:
Is E. coli Lon mutant viable?
Is E. coli FtsH mutant viable?
Is E. coli ClpP mutant viable?
Is E. coli Lon mutant viable?
Is E. coli FtsH mutant viable?
Is E. coli ClpP mutant viable?
==Lon is inviable==
==FtsH is inviable==
%%ClpP is viable (Fragile but viable)%%
==FtsH is inviable==
%%ClpP is viable (Fragile but viable)%%
43
New cards
Why is it important for intracellular protein degradation?
Managing essential processes and proteins functions
44
New cards
What enzymes (protease) are involved in intracellular protein degradation in E. coli?
* ClpXP or AP
* Lon
* FtsH
* Lon
* FtsH
45
New cards
What are the functions of tmRNA?
SsrA-tagged proteins are degraded by ClpX/P or ClpA/P proteases
46
New cards
What is the function of the ATP-dependent protease FtsH in E. coli?
Tags Membrane associated and soluable substrates
47
New cards
Which Proteins are subject to export?
Basically any protein not membrane bound
* cytoplasmic membrane proteins
* outer membrane proteins
* periplasmic proteins
* extracellular proteins, e.g., exoenzymes
* cytoplasmic membrane proteins
* outer membrane proteins
* periplasmic proteins
* extracellular proteins, e.g., exoenzymes
48
New cards
What proteins are on the cytoplasmic membrane and outer membrane as well as in the periplasm?
* Transporters
* Electron transporter chain proteins
* Periplasmic binding proteins/chaperons
* Porins
* Electron transporter chain proteins
* Periplasmic binding proteins/chaperons
* Porins
49
New cards
What is the function of YidC (insertase)?
1. directly inserts membrane proteins without signal peptide, e.g., M13 phage procoat protein.
2. works with the SRP system/SecYEG to insert most proteins into cytoplasmic membrane.
3. helps some membrane proteins, such as LacY (lactose permease), to fold correctly inside the membrane. It likely acts as a holding chaperone to facilitate folding.
50
New cards
Is a SecB-dependent signal peptide located at the N-terminus or C-terminus of the preprotein?
dependent signal peptide located at the N-terminus for degradation
51
New cards
Briefly compare the Sec and TAT systems in terms of the susbstrates, the signal peptide, whether coupled with translation, and the translocase.
Sec: Deals with unfolded proteins
Tat: Deals with folded proteins
Tat: Deals with folded proteins

52
New cards
What kind of proteins is mainly targeted by the SRP (signal recognition particle) system?
inner-membrane proteins with long+strongly hydrophobic signal peptides
53
New cards
Protein trafficking systems into or across cytoplasmic membranes of E. coli are…
* Sec
* SRP
* YidC
* Tat
* SRP
* YidC
* Tat
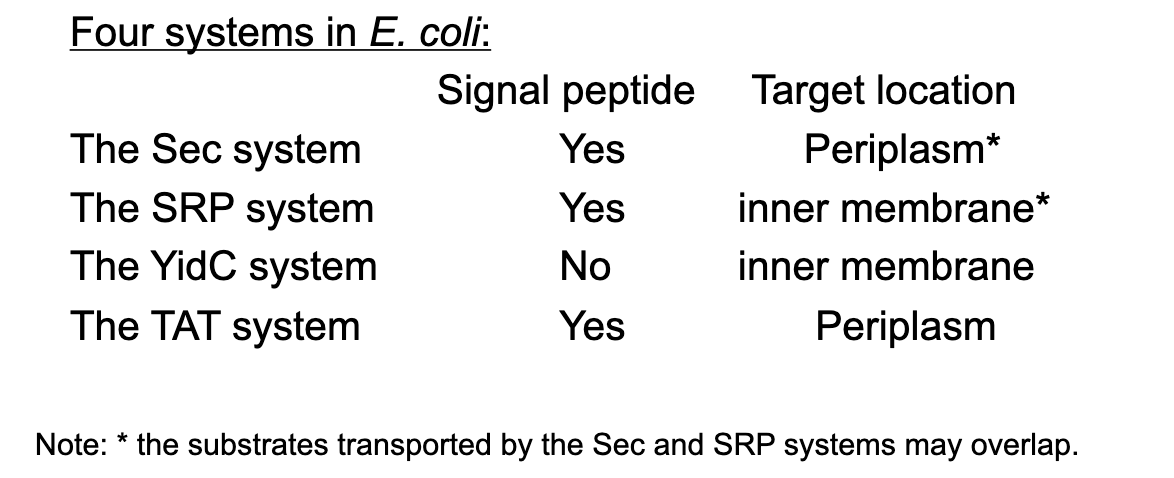
54
New cards
What is regulated by quorum sensing, and the secretion triggered at high cell density? And Why?
To ensure that the behavior they exhibit is coordinated and effective.
\
(caused by secreted proteins: Pectin lyase, Exo-poly-a-D-galacturonosidase, Pectin methylesterase, Cellulase)
\
(caused by secreted proteins: Pectin lyase, Exo-poly-a-D-galacturonosidase, Pectin methylesterase, Cellulase)
55
New cards
Type II signal for T2SS
Carry’s the N-terminal signal of N-degrons (Degradation signal carrier)
\
* Folding is a prerequisite.
* The signal is likely a conformational patch of the folded protein.
* In some cases, the signal is located immediately after the signal peptide at the N-terminus of the mature protein.
\
* Folding is a prerequisite.
* The signal is likely a conformational patch of the folded protein.
* In some cases, the signal is located immediately after the signal peptide at the N-terminus of the mature protein.
56
New cards
Type V Secretion System
Autotransporters (Self-catalyzed)
Intermembrane transporter reliant on the Sec system
Intermembrane transporter reliant on the Sec system
57
New cards
The Type VI system of Gram negative bacteria delivers toxins to other bacteria upon contact.
\
Which cellular components are the toxins targeting to damage?
\
Which cellular components are the toxins targeting to damage?
Cell membrane/Cell wall/Cytoplasmic membrane
58
New cards
Which type of secretion system transports pectin lyase from the plant pathogen Erwinia sp.?
T2SS (Sec pathway)
59
New cards
Where are proteins synthesized in bacteria?
Cytoplasm
60
New cards
Where is a signal peptide located on a secreted preprotein?
N-terminal end
61
New cards
Periplasmic proteins without prosthetic groups require which system for membrane trafficking?
The Sec System
62
New cards
Cytoplasmic membrane proteins without signal peptides require which system for membrane trafficking?
YidC
63
New cards
What proteins are not subject to membrane trafficking?
Cytoplasmic proteins
64
New cards
Which type of secretion system transports pectin lyase from the plant pathogen Erwinia sp.?
The Out system
\
Responsible for their secretion across outer membrane because mutation of the Out genes cause the accumulation of secreted proteins in the periplasmic space.
\
Responsible for their secretion across outer membrane because mutation of the Out genes cause the accumulation of secreted proteins in the periplasmic space.
65
New cards
Which secretion system is present only in Gram positive bacteria?
T7SS
66
New cards
The proteins with a signal peptide at the C-termini (CTD) are secreted by which secretion system?
T9SS
67
New cards
Which are the targets of T6SS toxins?
1. Cell wall
2. Cytoplasmic membrane
3. DNA
68
New cards
Which secretion system is similar to the type IV pilus?
T2SS
69
New cards
Why are bacteria with T6SS immune to their own toxins released by their system?
The bacteria carry antitoxins to their own toxins
70
New cards
Where do two component systems normally sense signals (inducers)?
On the periplasmic side of the cytoplasmic membrane
71
New cards
Which amino acid residue on the response regulator is phosphorylated?
Asp- Aspartic acid
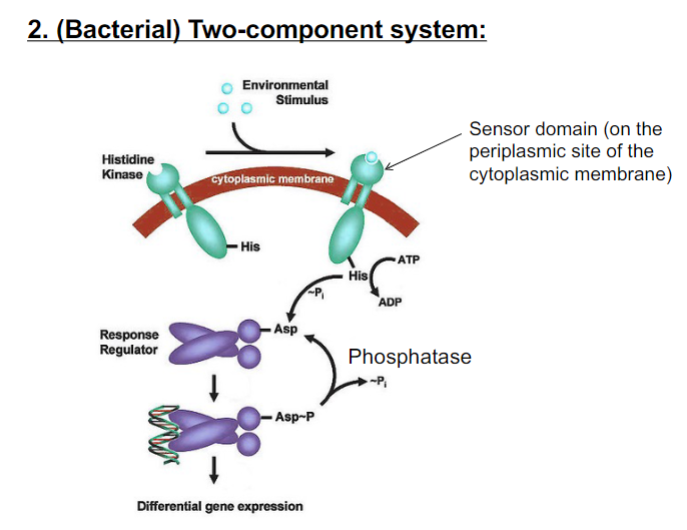
72
New cards
When is ArcB kinase 15% active?
When it forms one disulfide bond
73
New cards
Which operon is activated by NarP-P?
gene operon coding for nitrite reductase
74
New cards
What enzyme activity does NarX have when it senses nitrite?
Phosphatase that converts NarL-P to NarL + phosphate
75
New cards
Which amino acid residue ____________ is normally phosphorylated in the sensor/kinase, and which residue _____________________ is phosphorylated in the response regulator?
Histidine is phosphorylated on Histidine Kinase
\
Aspartic acid is phosphorylated on the Response regulator.
\
Aspartic acid is phosphorylated on the Response regulator.
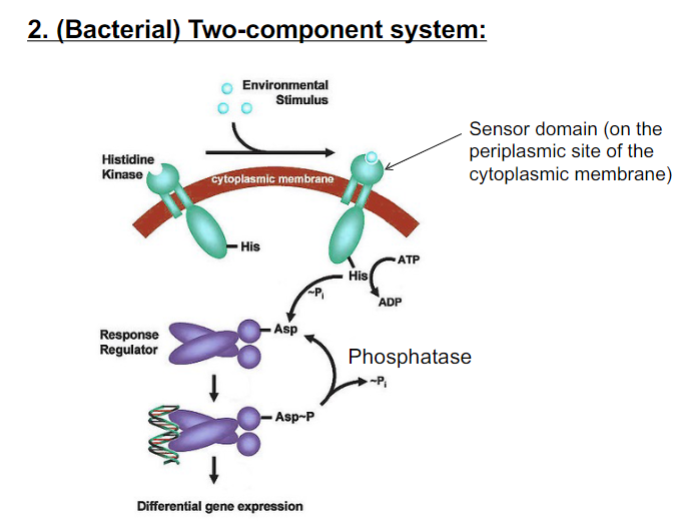
76
New cards
Bacteria senses chemical signals. When a signal is sensed on the cell surface, it is sensed by ___________________ systems. When a signal is transported into the cell and sensed in the cytoplasm, it is often sensed by __________________.
Sensor/His kinase: general structure
\
Response Regulator
\
Response Regulator
77
New cards
How is SoxR activated by superoxide?
O2- positivly charges \[2Fe-2S\]+→\[2Fe-2S\]2+
![O2- positivly charges \[2Fe-2S\]+→\[2Fe-2S\]2+](https://knowt-user-attachments.s3.amazonaws.com/6662401d88274abf851c256cb60b366a.jpeg)
78
New cards
Why is the nitrate reductase gene activated only in the presence of nitrate under anaerobic conditions?
Low to 0% O2 and nitrate presence activates NAR system
* specifically NarL-P and NarP-P
* specifically NarL-P and NarP-P
79
New cards
List three genes that are regulated by ArcB-ArcA.
* Cytochrome bd oxidase (cydAB): Cytochrome bd oxidase is a terminal oxidase that allows bacteria to respire under microaerophilic conditions. The expression of cydAB is upregulated by ArcB-ArcA in response to low oxygen levels, which helps the bacteria to maintain energy production in oxygen-limited environments.
\
* Fumarate reductase (frdABCD): Fumarate reductase is an enzyme involved in anaerobic respiration, and its expression is upregulated by ArcB-ArcA under anaerobic conditions. This allows bacteria to use fumarate as an electron acceptor when oxygen is not available.
\
* Glucose transporter (ptsG): The expression of the glucose transporter ptsG is downregulated by ArcB-ArcA in the presence of oxygen. This is because the bacteria can use aerobic respiration to generate more energy from glucose, and therefore do not need to take up as much glucose under oxygen-replete conditions.
\
* Fumarate reductase (frdABCD): Fumarate reductase is an enzyme involved in anaerobic respiration, and its expression is upregulated by ArcB-ArcA under anaerobic conditions. This allows bacteria to use fumarate as an electron acceptor when oxygen is not available.
\
* Glucose transporter (ptsG): The expression of the glucose transporter ptsG is downregulated by ArcB-ArcA in the presence of oxygen. This is because the bacteria can use aerobic respiration to generate more energy from glucose, and therefore do not need to take up as much glucose under oxygen-replete conditions.
80
New cards
What powers the rotation of bacterial flagella?
Proton Gradient
81
New cards
How fast can a bacterial flagellum rotate?
100,000 rpm
82
New cards
How does an attractant affect a bacterium?
Stimulate swim
83
New cards
Which protein is a sensor in chemotaxis?
MCP
84
New cards
Which of the following statement is incorrect?
\
* When Tar is partially methylated, it binds its attractant tighter than the fully methylated Tar.
\
* When Tar is unmethylated, it binds its attractant tighter than partially methylated Tar.
\
* When Tar binds its attractant, it decreases the CheA activity.
\
* None of these
\
* When Tar is partially methylated, it binds its attractant tighter than the fully methylated Tar.
\
* When Tar is unmethylated, it binds its attractant tighter than partially methylated Tar.
\
* When Tar binds its attractant, it decreases the CheA activity.
\
* None of these
None of them is incorrect
85
New cards
What is a Flagellum’s major structures?
* 20 different proteins
* Basal body
* Hook
* Filament
* Basal body
* Hook
* Filament
86
New cards
What are the major players in Chemotaxis?
* Attractants- decrease tumble, increase swim
* Repellents- increase tumble, decrease swim
* Repellents- increase tumble, decrease swim
87
New cards
What is the sensor and kinase of flagella?
Sensor: MCP
Kinase: CheA
Kinase: CheA
88
New cards
When CheB-P is activated, a methylesterase does what?
Removes a -CH3 from CheB-P
89
New cards
What is the methyltransferase for CheB-P?
CheR
90
New cards
Why is leucine an attractant at low concentrations, and a repellent at high concentrations to E. coli?
Due to a biphasic excitation
* Attracted to low concentrations
* Repelled by high concentrations
* Attracted to low concentrations
* Repelled by high concentrations
91
New cards
Chemotaxis consists of sensing and movement.
\
Briefly describe the bacterial response to an attractant (including signal transduction and flagella rotation direction).
\
Briefly describe the bacterial response to an attractant (including signal transduction and flagella rotation direction).
* Decrease Tumble
* Increase Swim
\
* MCP uses a two-component system to sense CheA and ATP→CheA-P reacts with CheY→CheA and CheY-P are produced→CheY-P interacts with switch and switches rotation to clockwise
* CheA-P=more swim
* CheY-P=more tumble
\
* Clock-wise flagella rotation
* Increase Swim
\
* MCP uses a two-component system to sense CheA and ATP→CheA-P reacts with CheY→CheA and CheY-P are produced→CheY-P interacts with switch and switches rotation to clockwise
* CheA-P=more swim
* CheY-P=more tumble
\
* Clock-wise flagella rotation
92
New cards
Bacteria can adapt (memory) to an attractant in chemotaxis.
\
Briefly describe the adaption model.
\
Briefly describe the adaption model.
* recognizes a common attractant or repellent
* then create positive or negative feedback loops to guide a bacteria’s chemotaxis.
* then create positive or negative feedback loops to guide a bacteria’s chemotaxis.
93
New cards
What is the Fenton reaction?
Fe2+ + H2O2 → Fe3+ + OH- + OH•
Primary purpose for making OH• radicals
Primary purpose for making OH• radicals
94
New cards
How is FNR inactivated?
Increased oxygen levels inactivate and cause disassociation of DNA activators
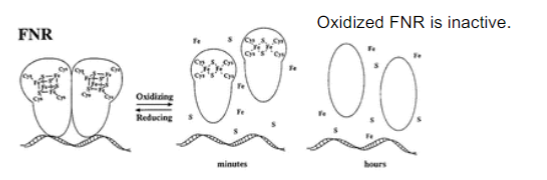
95
New cards
When E. coli cells encounter HCl in stomach, HCl enters into the cells via diffusion and dissociates into H+ and Cl-.
\
How does E. coli remove H+ inside the cell?
\
How does E. coli remove H+ inside the cell?
Superoxide Dismutase removes H+
96
New cards
How is RpoH (s32) activity inhibited at 30°C?
rpoH mRNA forms secondary structures that prevent translation.
97
New cards
Briefly describe the two ways that Dps protects DNA.
1. co-crystal formation at low Mg2+ concentrations DNA is compacted into nucleoids to protect from further damage
2. Use of iron ions to sequester and protect DNA from ROS
98
New cards
Under starvation, ppGpp is produced and accumulated.
\
What can ppGpp do?
\
What can ppGpp do?
mediates starvation stress responses
* efficient nutrient scavenge
* degradation of cellular components, RNA, proteins, lipids
* reduction in number of ribosomes
* condensation of chromosomal DNA for protection
* Reduce or shut down DNA replication
* reduce the synthesis of other cellular components proportionally
* efficient nutrient scavenge
* degradation of cellular components, RNA, proteins, lipids
* reduction in number of ribosomes
* condensation of chromosomal DNA for protection
* Reduce or shut down DNA replication
* reduce the synthesis of other cellular components proportionally
99
New cards
Describe the mechanisms of oxidative damage.
* ROS react with damaged cellular components (lipids, proteins, DNA)
* Inactivate some enzymes by reacting with their activation sites.
* Inactivate some enzymes by reacting with their activation sites.
100
New cards
Discuss how OxyR and SoxRS sense oxidative stress and their activated genes and the functions of the corresponding enzymes.
OxyR- activated by H2O2 and other oxidizing agents, binding to promoters responsible for oxidative stress response
SoxRS- activated by O2- and NO (nitric oxide), catalyzing superoxide breakdown of hydrogen peroxide and FNR reactions
SoxRS- activated by O2- and NO (nitric oxide), catalyzing superoxide breakdown of hydrogen peroxide and FNR reactions