1-Igneous Minerals and Processes
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
What is a mineral?
A naturally occurring, inorganic solid, with a characteristic chemical composition and a regular internal atomic arrangement which results in a tendency to develop external crystal faces
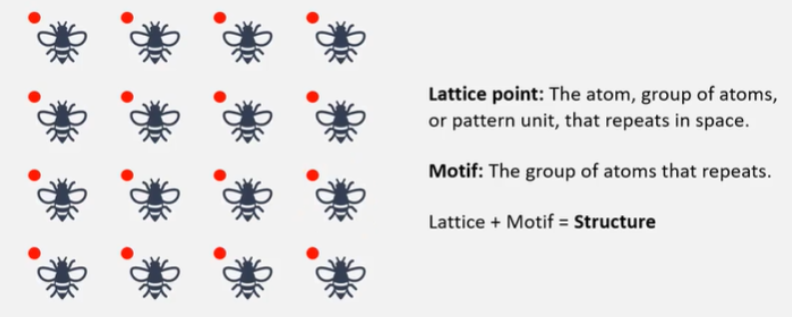
What is the difference between a lattice point and a motif?
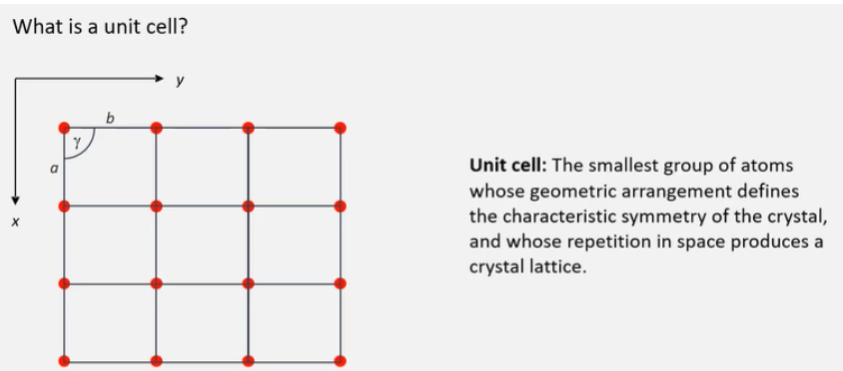
What is a unit cell?
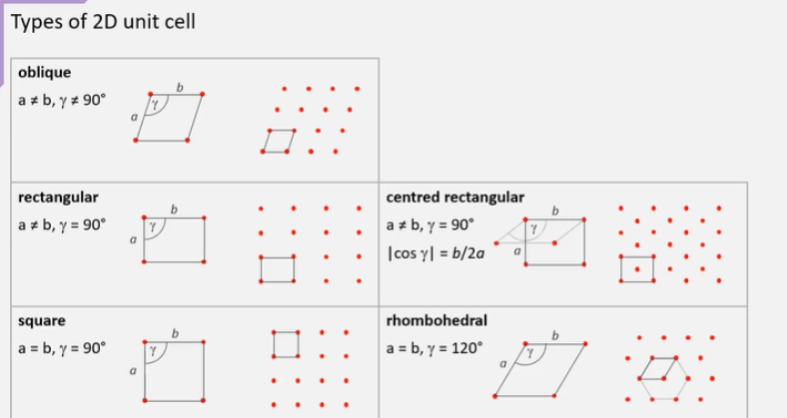
What does it mean by primitive v centred rectangular?
Primitive = unit cells only have lattice points only at their corners
Centred rectangular = has additional lattice points at its centre
Z = is the total number of formula units contained per cell
What are the types of 2D unit cells, don’t need names but general features?
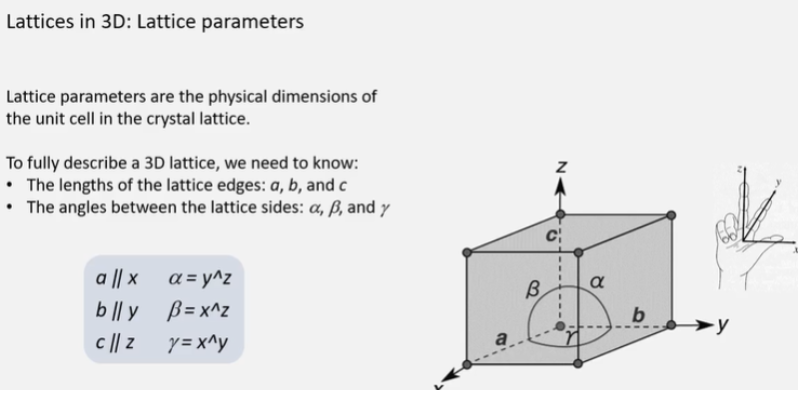
For the 3D lattice structure, where the the angles: alpha, beta, and gamma.
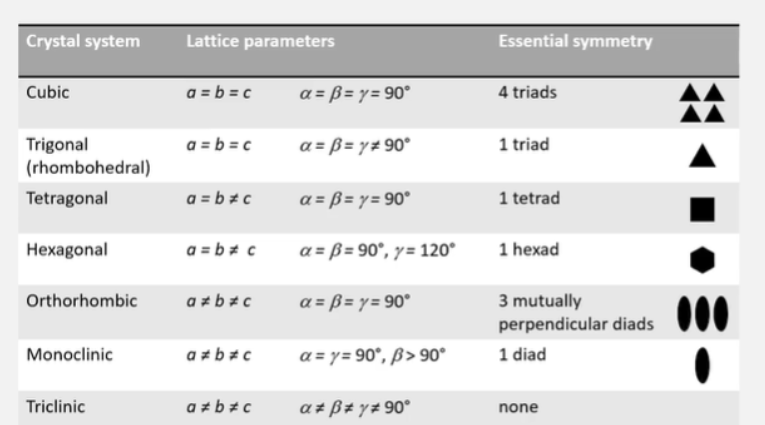
What are the seven crystal systems?
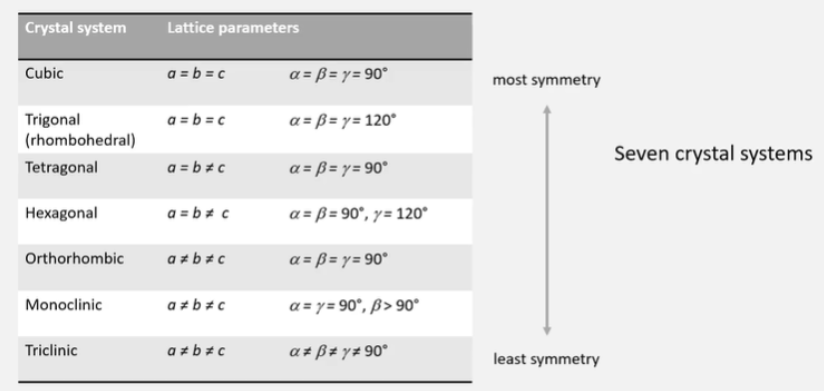
What are the five types of 3D unit cells?
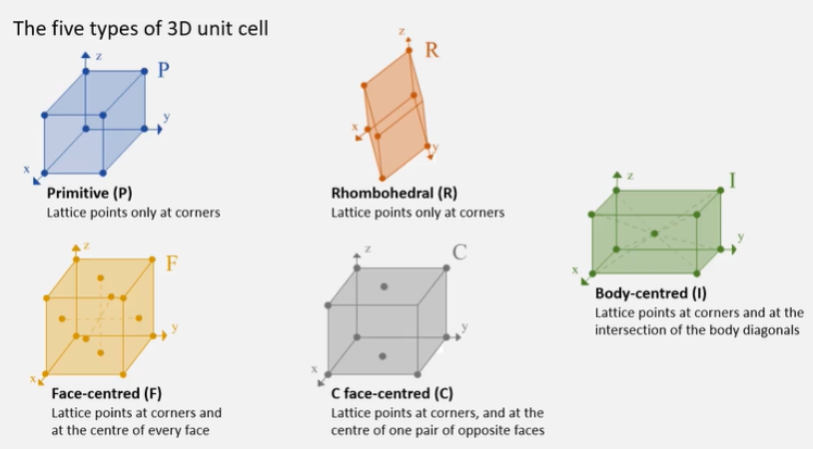
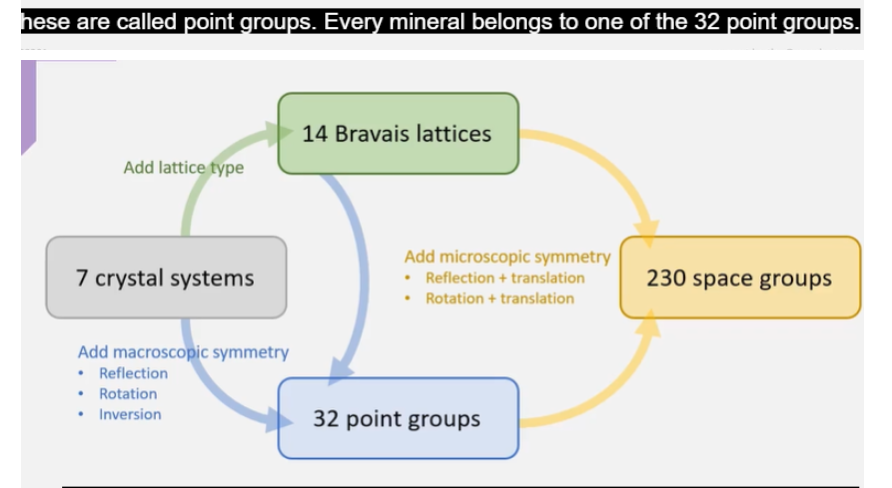
What is Bravais Lattice?
14 different ways
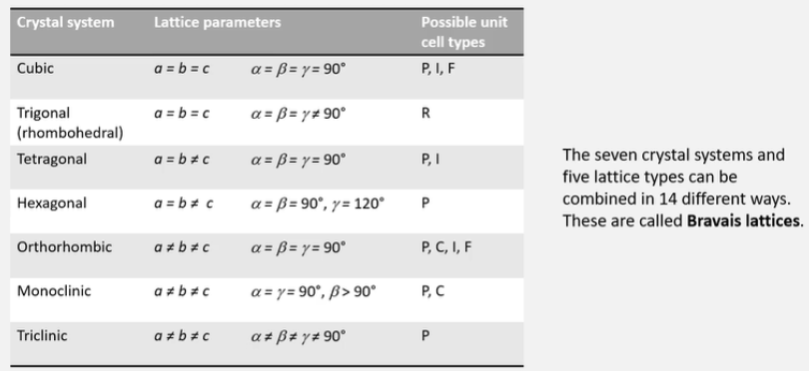
How many mirror planes are there in a cube?
9 mirror planes in a cube
What are the words for different orders of rotation?
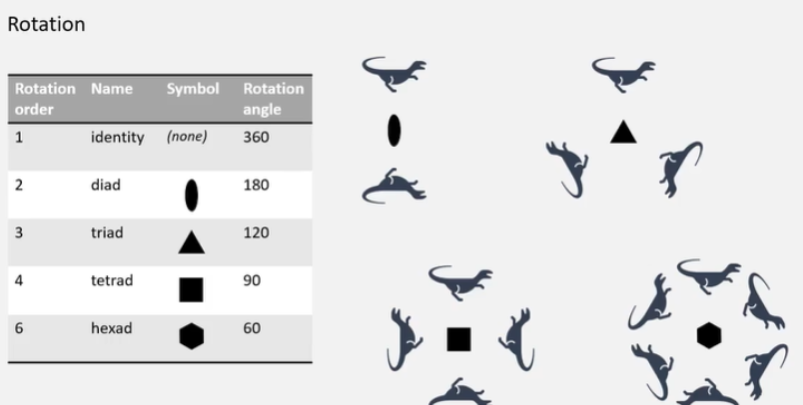
What is rotoinversion?
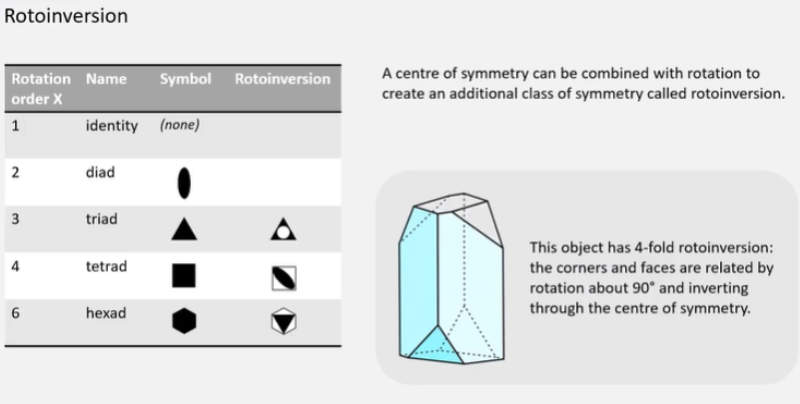
What is the essential symmetry for the 7 crystal systems?
Including macroscopic symmetry and microscopic symmetry how many point groups and space groups are there?
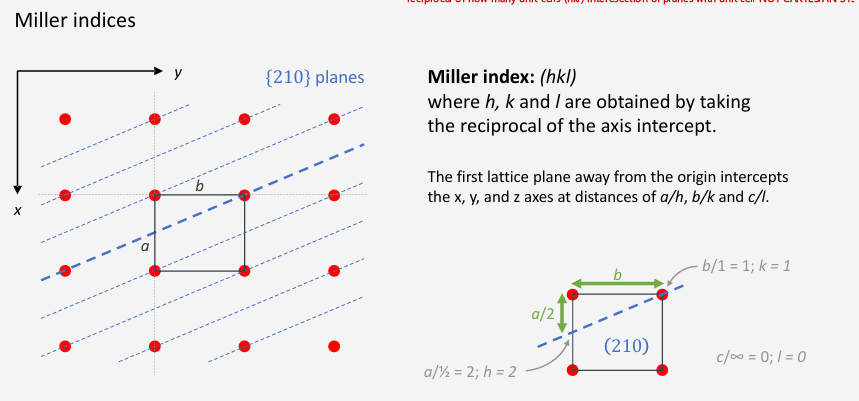
How do you work out the miller index (hkl)?
Reciprocal of how many unit cells it takes for the planes and the unit cell to intersect.
How many unit cells from origin, on the same plane
Normal bracket is one plane isolation
Curly bracket it for all planes with the same symmetry
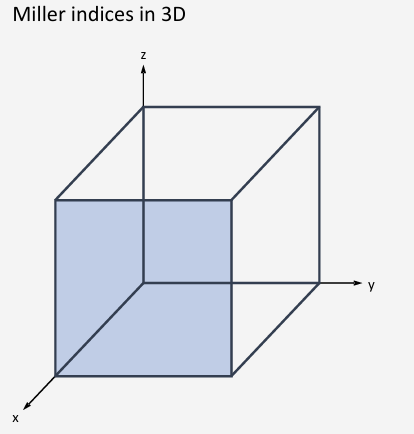


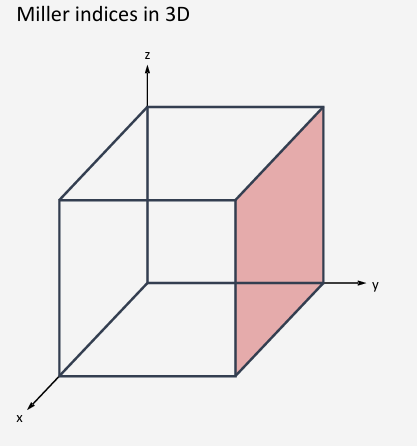
(010)
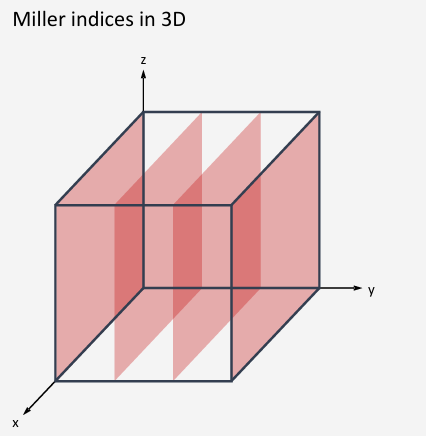

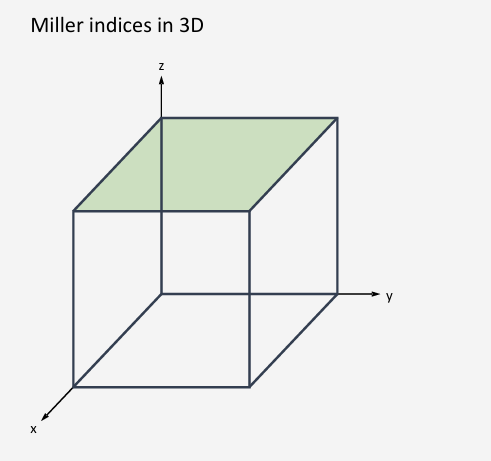

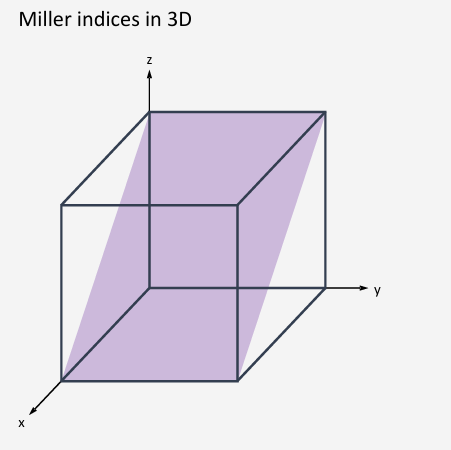

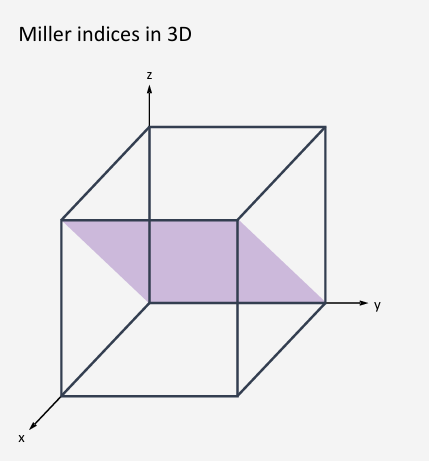
h=1 intercept with the x=axis at 1 unit, k=0 intercept with y-axis is infinite (plane is parallel to y-axis), l=-1 intercept with the z-axis is at -1 unit

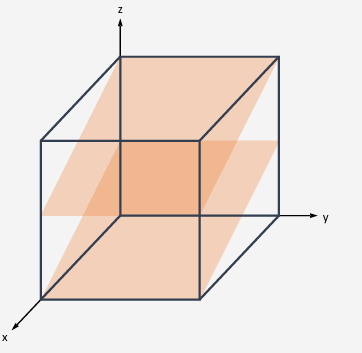

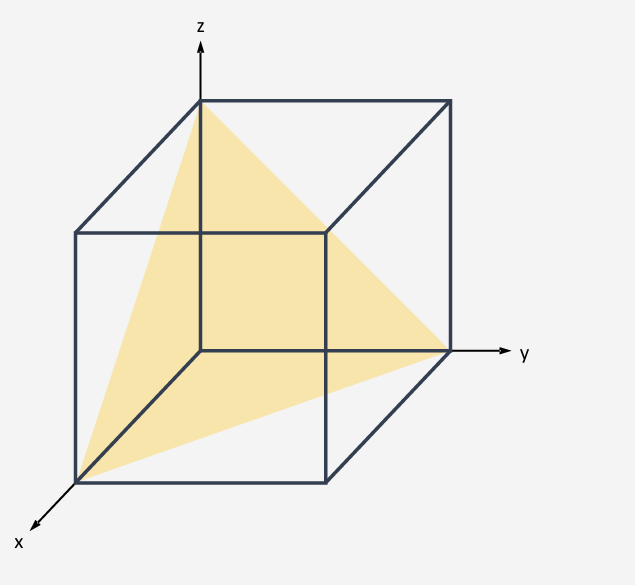
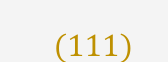

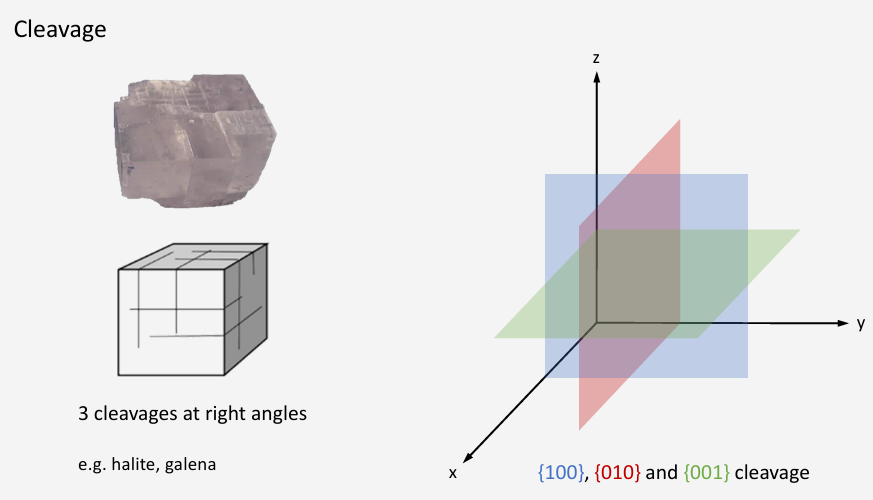
What is meant by cleavage?
Cleavage = controlled breakage along preferred planes.
Twinning = mirror-like or rotational symmetry in the crystal lattice, producing twin domains.
How many cleavage planes to biotite and muscovite have?
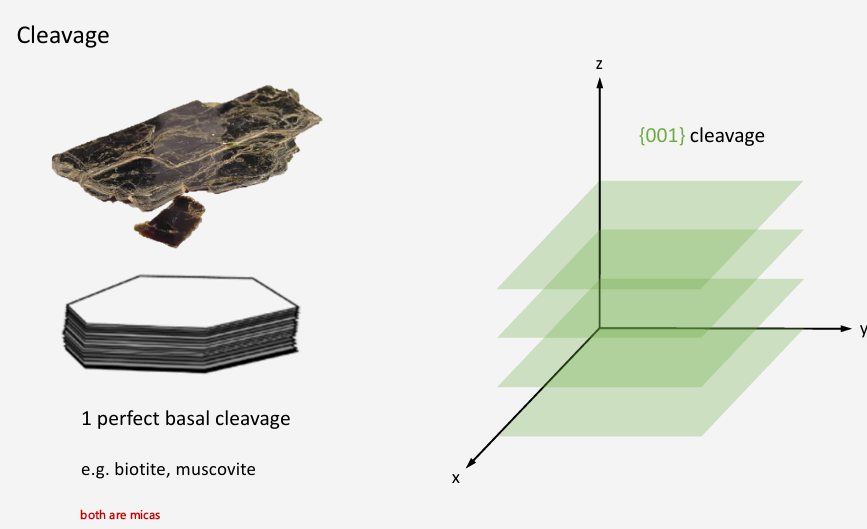
How many cleavage planes does halite and galena have?
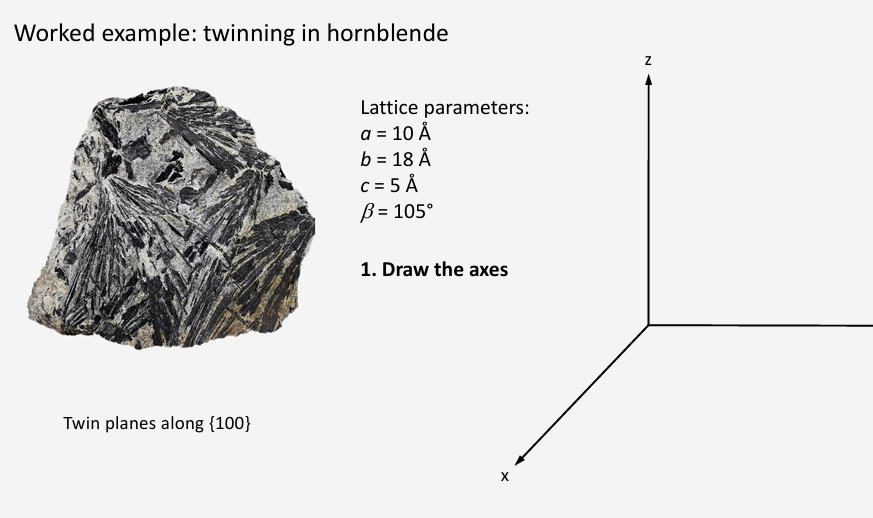
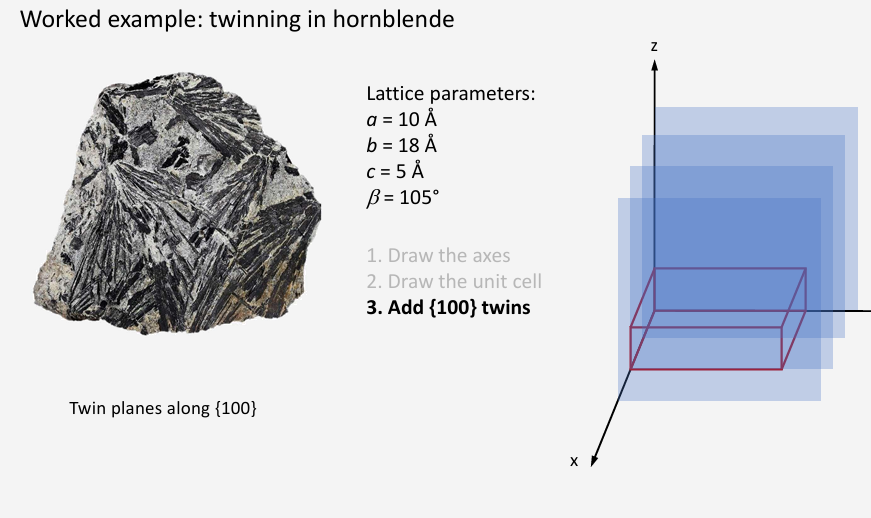
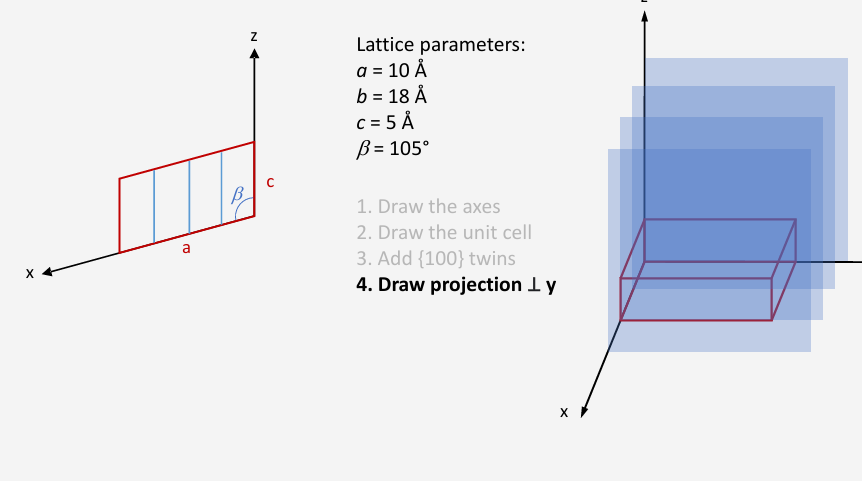
Draw projection along y axis
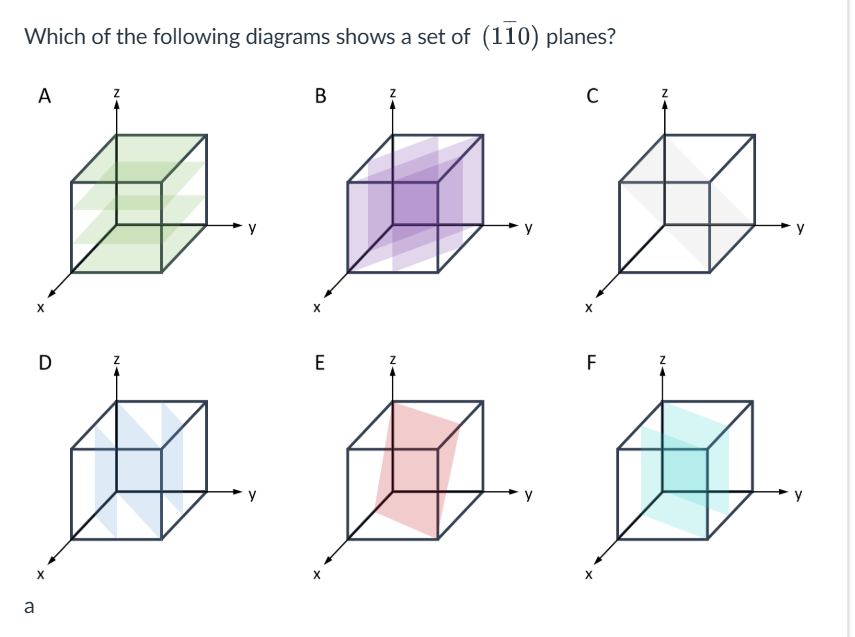
D
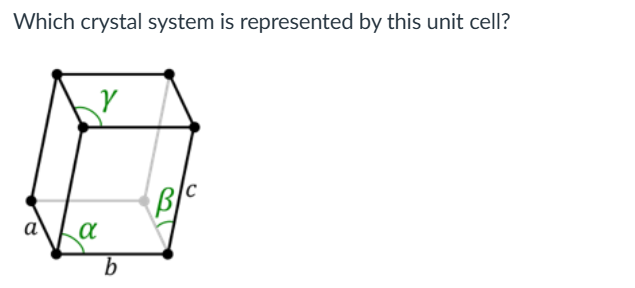
Triclinic
Which of these crystal systems has the least symmetry? Triclinic, trigonal, orthorhombic, monoclinic
Triclinic, triclinic crystal system has the least symmetry of all seven crystal systems.
What is the essential symmetry element of the tetragonal crystal system?
1 tetrad
What is true about unit cells?
unit must always reflect the symmetry of the lattice
must always contain an integer number of formula units
all unit cells have lattice points at their corners, some have additional lattice points in the centre of cell or on faces
What is the essential symmetry element of the cubic crystal system?
4 triads