Chemistry - Module 7 (Inspire)
1/41
Earn XP
Description and Tags
Based off Inspire Chem book
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
42 Terms
Lesson 1 Key Ideas:
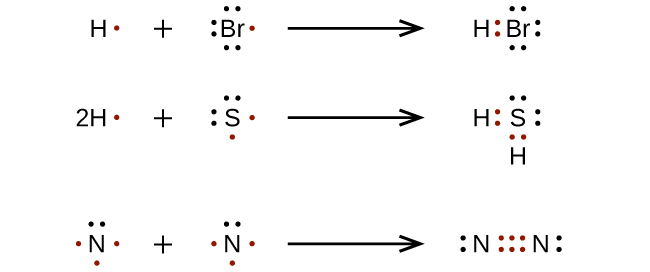
Covalent bonds form when atoms share one or more pairs of electrons.
Sharing one pair, two pairs, and three pairs of electrons form single, double, and triple covalent bonds, respectively.
Orbitals overlap directly in sigma bonds, parallel orbitals overlap in pi bonds. A single covalent bond is a sigma bond and pi bonds.
Bond dissociation energy is needed to bank a covalent bond.
Lesson 2 Key Ideas:
Names of covalent molecular compounds include prefixes for the number of each atom present. The final letter of the prefix is dropped if the element name begins with a vowel.
Molecules that produce H+ in solution are acids. Binary acids contain hydrogen and one other element. Oxyacids contain hydrogen and an oxyanion.
Lesson 3 Key Ideas:
Different models can be used to represent molecules.
Resonance occurs when more than one valid Lewis structure exists for the same molecule.
Exceptions to the octet rule occur in some molecules.
Lesson 4 Key Ideas:
VSPER model theory states the electron pairs repel each other and demonstrate both the shape of and bond angles in a molecule.
Hybridization explains the observed shapes of micelles by the presence of equivalent hybrid orbitals.
Lesson 5 Key Ideas:
The electronegativity difference determines the character of a bond between atoms.
Polar bonds occur when electrons are not shared equally, forming a dipole.
The spacial arrangement of polar bonds in a molecule determines the overall polarity of a molecule.
Molecules attract each other by weak intermolecular forces. In a covalent network solid, each atom is covalently bonded to other atoms.
mono
one
di
two
tri
three
tetra
four
penta
five
hexa
six
hepta
seven
octa
eight
nona
nine
deca
ten
CHNOPS
carbon, hydrogen, nitrogen, oxygen, phosphorus, sulfer
Diatomic Molecules
Sharing/covalent
H2, O2, and N2
single, double, triple bonds
Halogens
F2, Cl2, Br, I2
Ionic or Covalent:
PH3: Covalent
NaCl: Ionic
CO2: Covalent
Cu(SO4): Ionic
SiH4: Ionic
Following naming terms
CO = carbon monoxide
CO2 = carbon dioxide
SO2: sulfer monoxide
NF3 = nitrogen trifluoride
CCl4 = carbon tetrachloride
As2O3 = diarsenic trioxide
N2S = dinitrogen monosulfide
Li3N = lithium nitride
Following naming terms to be known:
P2Br4 = diphosphorous tetrabromide
SiO2 = silicon dioxide
SF6 = sulfide tetrafluoride
PH3 = phosphorous trihydride
NO2 = nitrogen dioxide
Covalent bond
A bond that forms when atoms share one or more pairs of electrons.
Molecule
A group of two or more atoms bonded together, representing the smallest fundamental unit of a chemical compound.
Lewis Structure
A diagram that represents the arrangement of valence electrons among atoms in a molecule, showing how they are bonded.
Sigma Bond
A type of covalent bond formed by the direct overlap of atomic orbitals, allowing for electron sharing between atoms.
Pi bond
A type of covalent bond formed by the lateral overlap of atomic orbitals, resulting in electron sharing in addition to a sigma bond. This bond typically occurs in double and triple bonds between atoms.
endothermic reaction
A chemical reaction that absorbs energy, usually in the form of heat, from its surroundings, leading to a temperature decrease in the environment.
exothermic reaction
A chemical reaction that releases energy in the form of heat or light, often resulting in a temperature increase of the surroundings.
oxyacid
An acid that contains oxygen, hydrogen, and another element, typically releasing hydrogen ions in aqueous solutions.
structural formula
A representation of a molecule that shows the arrangement of atoms and the bonds between them, often using lines to depict bonds.
resonance
A concept in chemistry describing the delocalization of electrons in molecules where bonds can be represented as an average of multiple structures. It illustrates how the molecule can exist in different forms without changing the overall composition.
Coordinate Covalent bond
A type of covalent bond in which both electrons shared in the bond are contributed by one of the atoms.
VSPER model
A theory used to predict the geometry of individual molecules based on the repulsion between electron pairs around a central atom. It stands for Valence Shell Electron Pair Repulsion.
Hybridization
A concept that describes the mixing of atomic orbitals to form new hybrid orbitals, which can be used to form covalent bonds in molecules.
Polar Covalent bonding
A type of covalent bond where electrons are unequally shared between atoms, resulting in a partial positive charge on one atom and a partial negative charge on the other.
How do atoms bond in covalent bonds?
Atoms bond in covalent bonds by sharing one or more pairs of electrons to achieve stable outer electron shells.
How do you name covalent molecules?
Covalent molecules are named using prefixes to show the number of each atom and ending the second element with “-ide.”
Example:
CO₂ → carbon dioxide
N₂O₅ → dinitrogen pentoxide
How are electrons shared in covalent compounds?
In covalent compounds, electrons are shared between atoms so each atom can fill its outer energy level and become stable.
What shapes do molecules form?
Molecules form different shapes based on how electron pairs repel each other around the central atom. Examples include:
Linear: CO₂ (carbon dioxide)
Bent: H₂O (water)
Trigonal planar: BF₃ (boron trifluoride)
Tetrahedral: CH₄ (methane)
How does the shape of. a molecule affect how it is held?
The shape of a molecule affects how it is held because it determines how molecules interact and bond with each other — for example, through polarity and attraction forces. Molecules with uneven shapes (like water) are polar and stick together strongly, while symmetrical molecules (like CO₂) are nonpolar and have weaker attractions.
Lewis Dot Diagram examples: