Topic 4 - Intermolecular Forces and the States of Matter: Intermolecular Forces
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
Intermolecular Forces
Additional forces (non-bonding attractions/repulsions between particles) that act when two substances are mixed; weaker than intramolecular bonds.
Set physical properties (solid/liquid/gas at room temperature, miscibility/solubility).
Electrostatic in nature — formed through interactions between positive (+) and negative (–) regions.
Strength determined by molecule/ion's ability to form/maintain dipoles (permanent or temporary).
Strength from weakest to strongest:
Dispersion forces (aka London dispersion forces);
Dipole-dipole forces;
Hydrogen bonding (extreme form of dipole-dipole forces).
All are much weaker than covalent/ionic bonds (e.g., melting/vaporising water needs far less energy than breaking O–H bonds), but cumulative effects can be large.
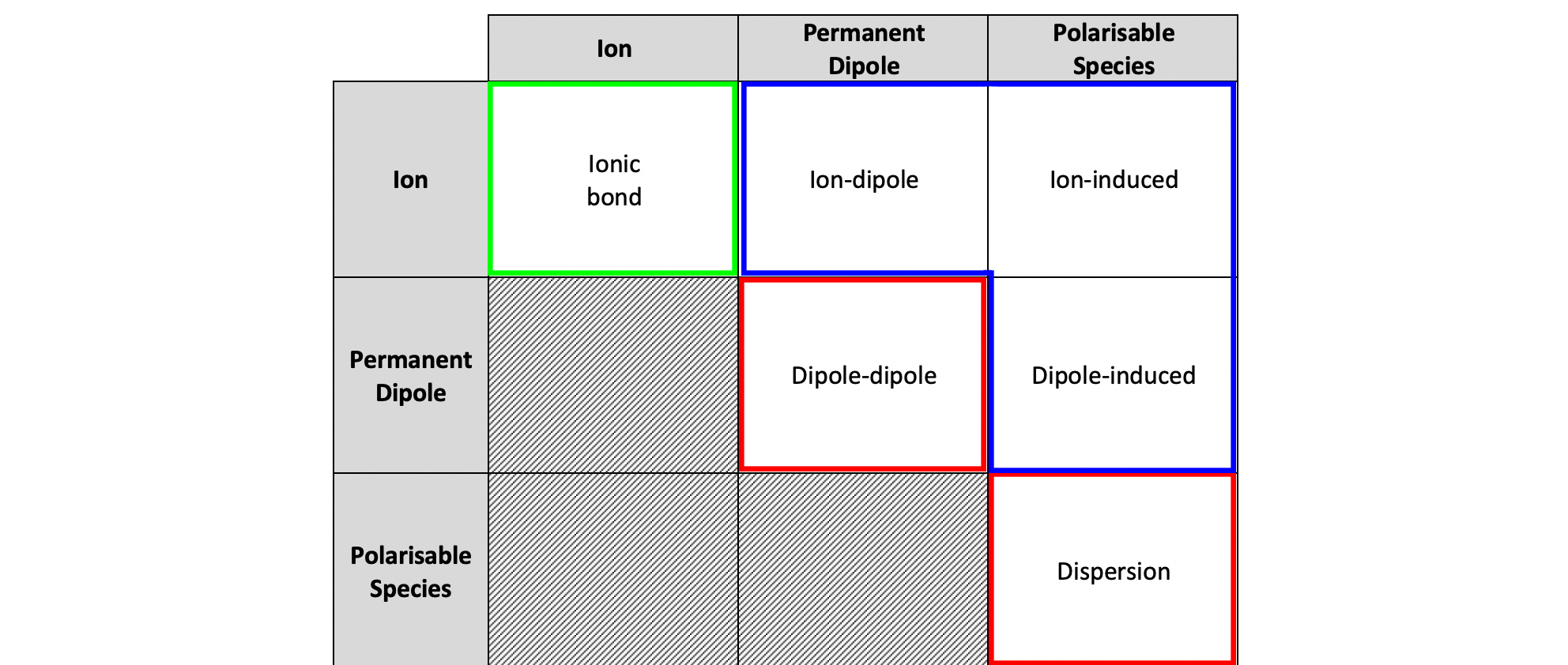
Dispersion Forces - Origin and Mechanism
Dispersion forces are present between ALL molecules and other atoms, ions or molecules.
Electron clouds fluctuate → momentary uneven electron distribution → instantaneous dipole (areas of positive and negative partial charge).
This can induce an opposite dipole in a neighbour → attraction.
Attractive alignments last longer than repulsive ones → net attraction.
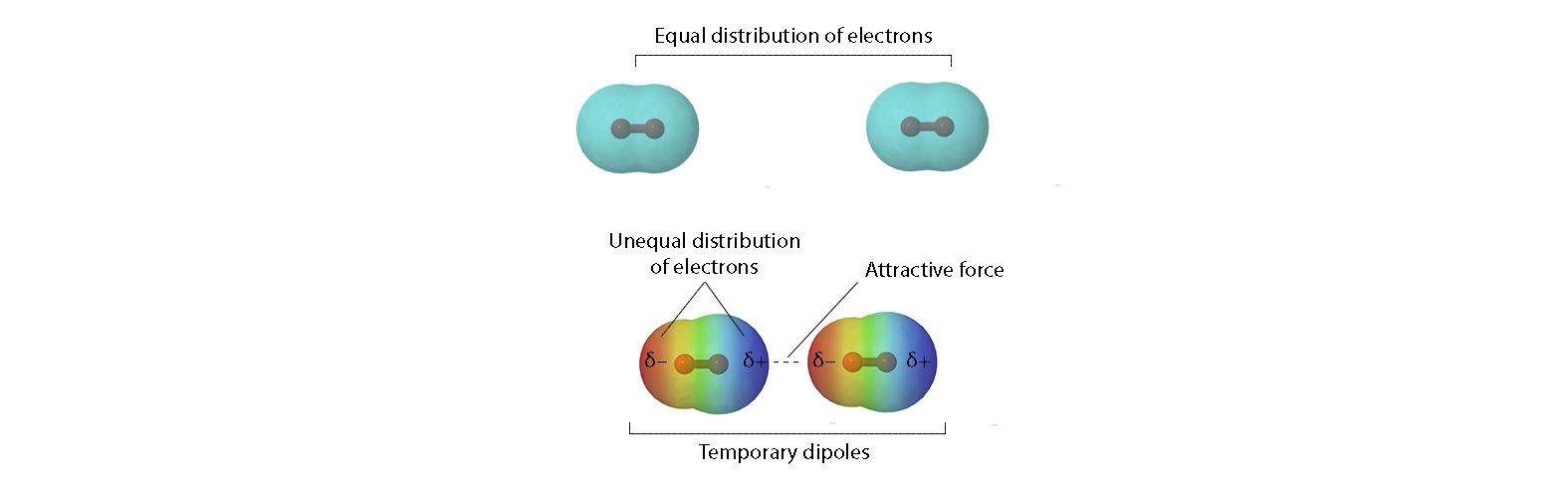
Dispersion Forces - Strength
Dispersion forces are present between ALL molecules and other atoms, ions or molecules.
What controls their strength
Number of electrons (size of e⁻ cloud):
More electrons → bigger, more distortable cloud → larger instantaneous dipoles → stronger dispersion.
Polarisability (ease of e⁻ cloud distortion): Increases with larger electron clouds and with shape.
Linear/extended molecules → larger, extended surface area → electron farther away from the nucleus → weak electron-nucleus attraction → fluid cloud → more easily polarised → stronger dispersion.
Compact/branched molecules → smaller contact area → electron closer to the nucleus → strong electron-nucleus attraction → stiff cloud → less polarizable → weaker dispersion.
Dispersion Forces - Property Trends
Dispersion forces are present between ALL molecules and other atoms, ions or molecules.
Boiling/melting points rise as IMFs strengthen because more heat is needed to overcome attractions.
Alkanes: Longer chains (larger clouds) and more linear shapes → higher boiling points than shorter/branched isomers.
Dipole–dipole Forces
Who has them: Interaction between polar molecules (permanent dipoles with δ+ / δ–).
Charge separation by strength: temporary (dispersion) < permanent dipole separation < ionic.
Mechanism: Close approach between polar molecules allows alignment (δ+ near δ–) → attraction; net effect is attractive overall.
Relationship to dispersion: Occur in addition to dispersion and are typically stronger than dispersion for small molecules.
Hydrogen Bonding
A type of chemical bond that occurs when a hydrogen atom, which is covalently bonded to an electronegative atom (like oxygen, nitrogen, or fluorine), is attracted to another nearby electronegative atom. An extreme form of dipole-dipole interaction.
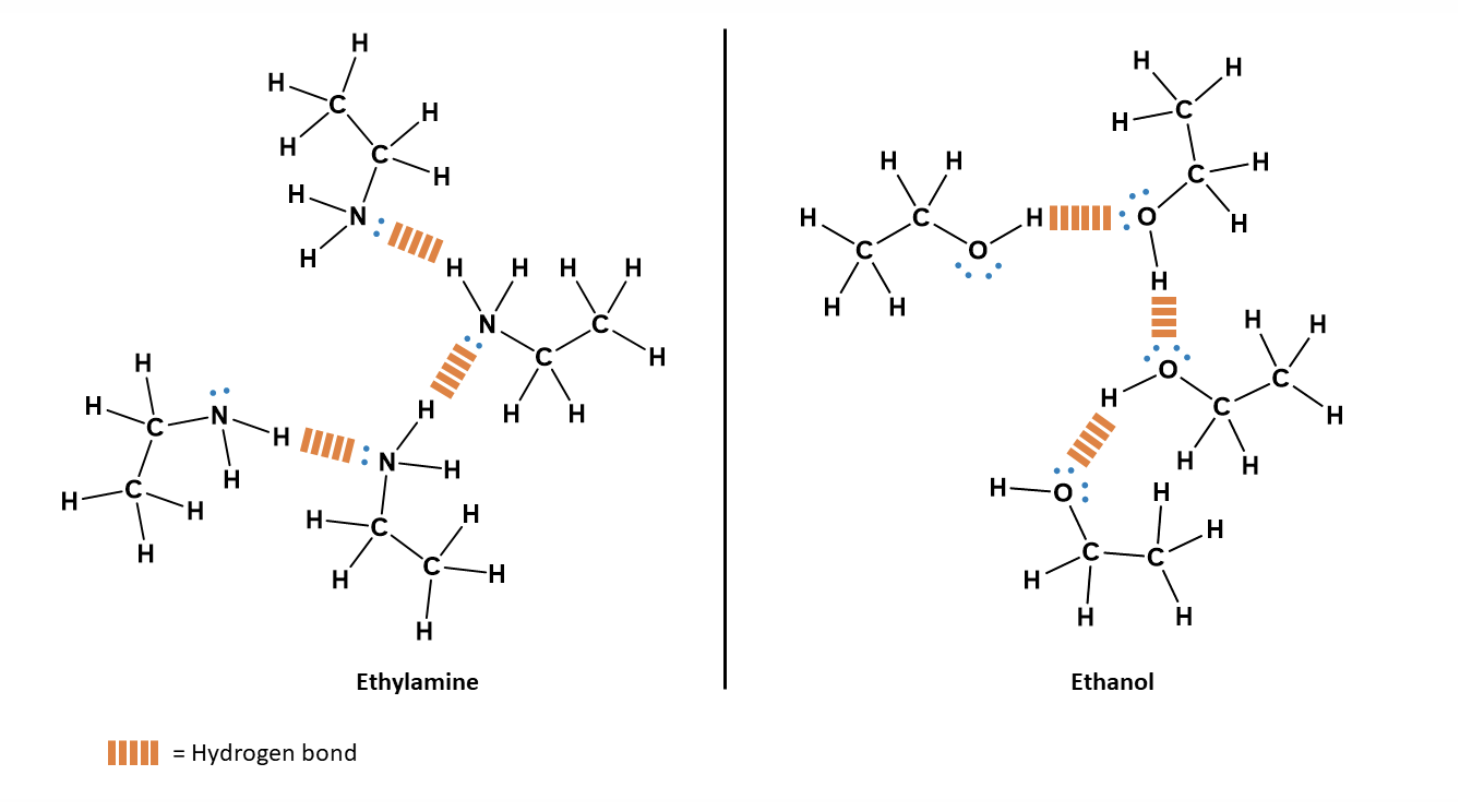
Hydrogen Bonding - Conditions for its Formation
Hydrogen Bond Donor
H atom must be covalently bonded directly to a highly electronegative atom (N, O, or F).
This creates a strong permanent dipole with the H atom becoming partially positive (δ+).
The δ+ hydrogen is available for attraction.
Hydrogen Bond Acceptor
Must be a strongly electronegative atom (N, O, or F) with one or more lone pairs of electrons.
If there are multiple electronegative atoms in a molecule to be chosen as the hydrogen bond acceptor, the most electronegative atom is likely to be the one
Does not require a hydrogen attached to it.
The lone pairs interact with the δ+ hydrogen of the donor.
Nature of H-Bonds
Strength: ~1/10 of a covalent bond.
Easily broken/reformed
Represented by dashed lines between donor and acceptor.
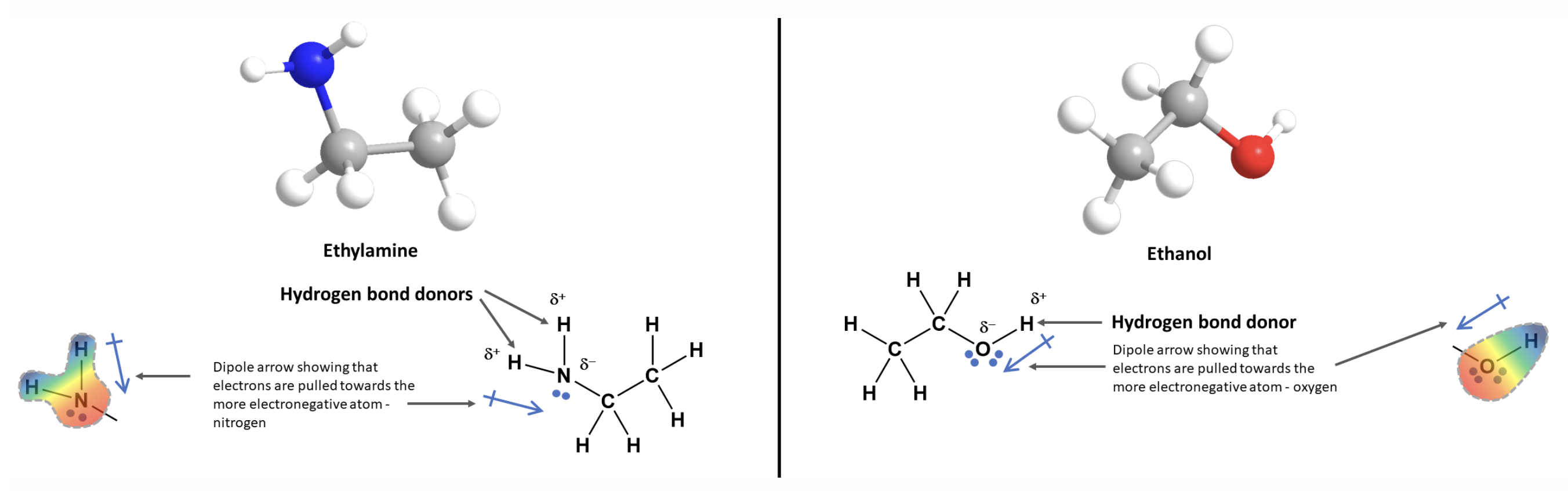
Hydrogen Bonding - Strength
Why are H-bnds so strong?
Hydrogen has only one electron, leaving its nucleus exposed when bound to N, O, or F.
Allows very close approach of electronegative atoms → unusually strong dipole-dipole interaction.
Intermolecular Forces & Solubility - General Rule
“Like dissolves like.”
Polar solvents dissolve polar solutes.
Non-polar solvents dissolve non-polar solutes.
Polar & non-polar do not mix.
Intermolecular Forces & Solubility - Mixing Process
For solute to dissolve:
Solute–solute and solvent–solvent interactions must be disrupted.
Replaced by solute–solvent interactions (intermolecular forces) that are equal to or stronger.
Solute
Substance being dissolved (minor component).
Solvent
Substance doing the dissolving (major component).
Soluble
Capable of dissolving.
Miscible
When two liquids dissolve in all proportions to form one phase.
In the case of a liquid being soluble in another liquid, the term miscible is often used in place of soluble.