ORGMED LAB: synthesis of acetylsalicylic acid
1/27
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
Aspirin (acetylsalicylic acid) is a _______________ substance.
white, crystalline solid
Aspirin is usually odorless, but develops a __________________ (odor) when exposed to moisture due to hydrolysis into __________________
vinegar-like odor of acetic acid
salicylic and acetic acid
Acetylsalicylic acid has a melting point of:
________°C
________°F
136
277
________________ is known to be one as one of the most common a nonsteroidal anti-inflammatory drug (NSAID) used to reduce pain, fever, and inflammation, and as an antithrombotic.
acetylsalicylic acid
acetylsalicylic acid can also be given (BEFORE / AFTER) taking _________, an anti-tubercular drug to prevent flushing reactions.
isoniazid
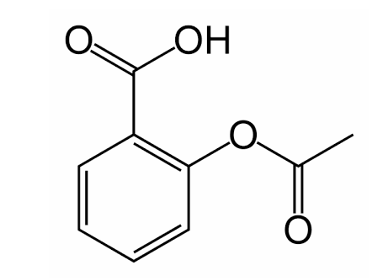
name this structure
acetylsalicylic acid
In aspirin, it is believed that the primary mechanism of action is the _____________ which results in the inability to synthesize prostaglandins, prostacyclins, and thromboxane.
irreversible acetylation of cyclooxygenase
as a result of the mechanism of action of aspirin, the pyrogenic effect of prostaglandins on the centers of thermoregulation and sensitive nerve endings is (INCREASED / REDUCED), which leads to a (ENHANCING / LESSENING) of sensitivity to painful neurotransmission
reduced
lessening
The antiaggregatory effect of aspirin is explained by the irreversible inability to synthesize __________ in the thrombocytes.
thromboxane A2
Aspirin can cause:
____________ reactions
_____toxicity
__________ stools
________, _______
and most commonly: ____________
hypersensitivity
ototoxicity
bloody or tar-like stools
rashes, fever
stomach ulcers
Gastric irritation due to aspirin can be caused by (HIGH / LOW) doses of aspirin
high
Gastric irritation due to aspirin can be caused by (HIGH / LOW) doses of aspirin
Gastric irritation due to aspirin can be caused by (CONCOMITANT / SEQUENTIAL) use with other drugs like other NSAIDs, corticosteroids and anticoagulants like ticagrelor, prasugrel, and clopidogrel
concomitant (sabay)
Gastric irritation due to aspirin can be caused by triple antithrombotic therapy of the drugs: _______, ________, ________
aspirin
clopidogrel
warfarin
Gastric irritation due to aspirin can be caused by infection of this microorganism: ___________
Helicobacter pylori
Gastric irritation due to aspirin can be caused by history of _________ or ___________
peptic ulcer or gastric bleeding
Gastric irritation due to aspirin can be caused by (YOUNGER / OLDER) age
older
Gastric irritation due to aspirin can be caused by presence of ___________________
other severe comorbidities
Aspirin must not be given to children recovering from (BACTERIAL / VIRAL) infection,
as it can cause ______________ (swelling in the liver and brain)
Reye’s syndrome
Aspirin was synthesized by the (TURKISH / GERMAN) chemist: _______________ at the end of the ___th century
german chemist
felix hofmann
19th century
the initial preparation of aspirin consists of an ____________ reaction catalyzed by _________ where salicylic acid is treated with ____________ gives acetylsalicylic acid.
esterification reaction
acid
acetic anhydride
in the reaction of salicylic acid with acetic anhydride, a hydroxyl group is converted into an __________, with _________ as byproduct
ester
acetic acid
when aspirin is reacted with FERRIC CHLORIDE solution, it will form ___________________
no color change
aspirin elicits no color change in ferric chloride test due to lacking a __________ group
phenol group
when aspirin is reacted with SODIUM HYDROXIDE solution, it will undergo ________ reaction, forming ________________
neutralization
sodium acetylsalicylate and water
A ______ (normality) sulfuric acid solution can be used to neutralize the excess NaOH and to determine the amount of aspirin present through titration.
2N sulfuric acid
___________ must be stored at room temperature and away from excess heat and moisture, and any vinegar odor-like smell that may produce from the medication must be disposed immediately.
aspirin
Fill in the blanks with the correct step number in synthesizing aspirin from salicylic acid.
___ Add carefully 5 to 10 drops of 85% phosphoric acid to the flask and swirl to mix thoroughly.
___ Weigh 3g of salicylic acid and place it in a 250 ml Erlenmeyer flask.
___ Add another 20 ml of water and cool in an ice bath. Scratch the walls of the flask with a stirring rod to induce crystallization.
___ Measure 6 ml of acetic anhydride and add it to the flask. (Note: Do this inside the hood and wear goggles.)
___ Filter the solid aspirin through a piece of pre-weighed filter paper using a funnel.
___ Weigh the product and calculate the percentage yield.
___ Heat the mixture for about 10 minutes in a beaker with warm water (78-80°C).
___ Wash the crystals with 2-3ml chilled water.
___ Add cautiously 20 drops of distilled water.
___ Place the filter with the product on a watch glass and put it in the oven at 100°C for about 30 minutes until dry.
3
1
6
2
7
10
4
8
5
9