sugars 2
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
describe the degradation of polymers of glucose
uses glycoside hydrolases
these perform nucleophilic substitution of -OR with -OH
they perform hybrid sn1/sn2 reactions
form an intermediate with carbocation characteristics
active site positions functional groups to control product chirality
whats the difference between inverting and retaining b-glycosidases
retaining keeps the alpha/ beta configuration of the -OR group when substituting it with -OH
inverting changes the configuration
different forms can interconvert, but slowly, so its still worth using different enzymes to control which type arises

describe the inverting mechanism of a b-glycosidase
-OR group takes a hydrogen from an acidic amino acid residue of the active site, and leaves, producing an oxocarbenium ion
water molecule approaches from the opposite side of where -OR left, and attacks the positive carbon, while a hydrogen is taken from this water molecule by a COO- amino acid from the active site
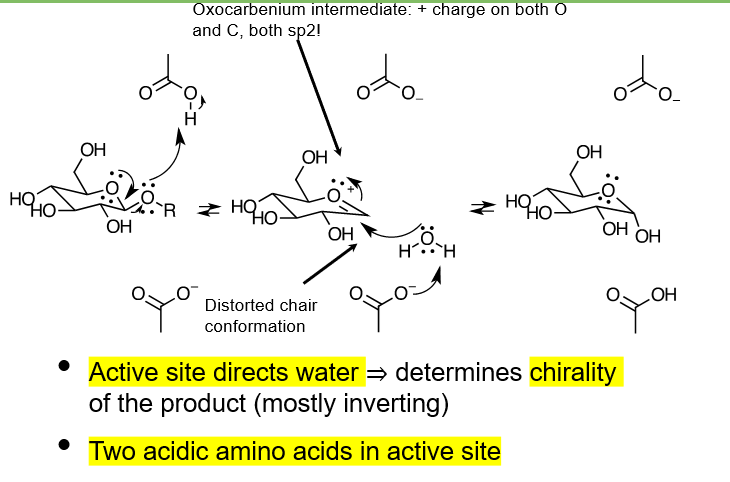
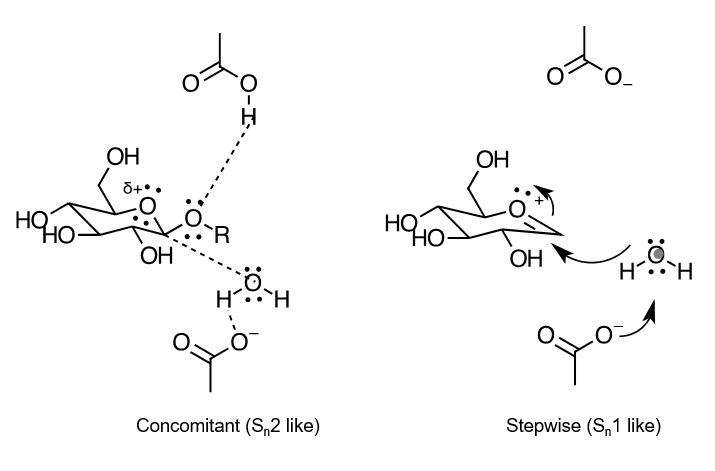
does the reaction described undergo sn1 or sn2 mechanism?
we arent sure, maybe should learn both. sn2 is where all steps happen at once, sn1 is stepwise
more likely sn1 though since theres a carbocation intermediate formed
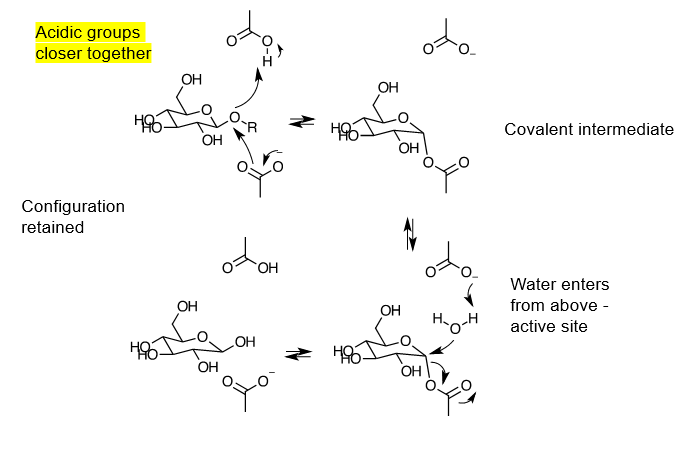
describe the retaining mechanism of b-glycosidases
acidic groups in active sites are closer together, and there is no space for water at first. this is the only difference in the enzyme structures
once oxocarbenium ion is made (same way as it is for inverting), aspartate attacks it to make a covalent intermediate
leaving of -OR makes space for water to enter, and aspartic acid that became aspartate (when giving OR its proton) will act as a base to deprotonate water, making -OH to attack carbon and break off the covalently bonded aspartate
what do glycosyl transferases do?
catalyse transfer of activated sugar to acceptors such as alcohols on other sugars, proteins, lipids or nucleic acids
how are sugars activated?
through coupling to nucleotides, hence making nucleotide sugars
once a transfer has occurred, a base is left behind
describe glycogen structure
glycogen has only one reducing end (all other ends are non-reducing because they cant ring open
branch points via a-1.6 linkages, result in glucose monomers linking to 3 others rather than 1 or 2
the one reducing end needs to be protected sometime
branchpoints mean that you can have fast oligomerisation or degradation
describe glycogen breakdown
breakdown occurs at non-reducing ends, which is why its good to have so many
uses phosphate rather than H2O to replace a linkage, producing glucose-1-phosphate which cant ring open and is non-reducing
G1P substitutes pyrophosphate component of UTP (activated by inorganic pyrophosphotase)
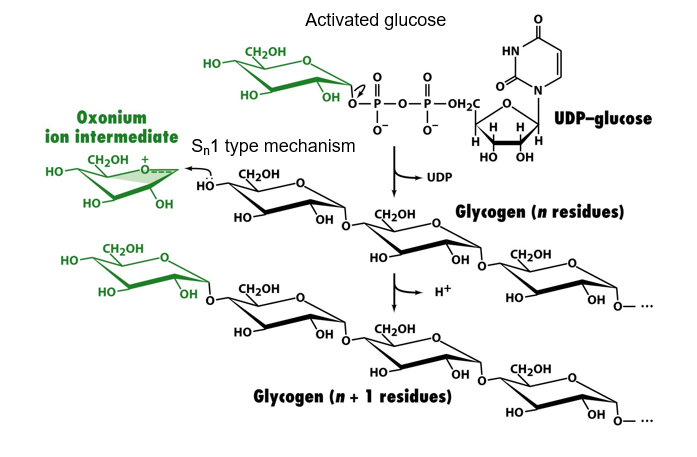
UDP-glucose is activated glucose
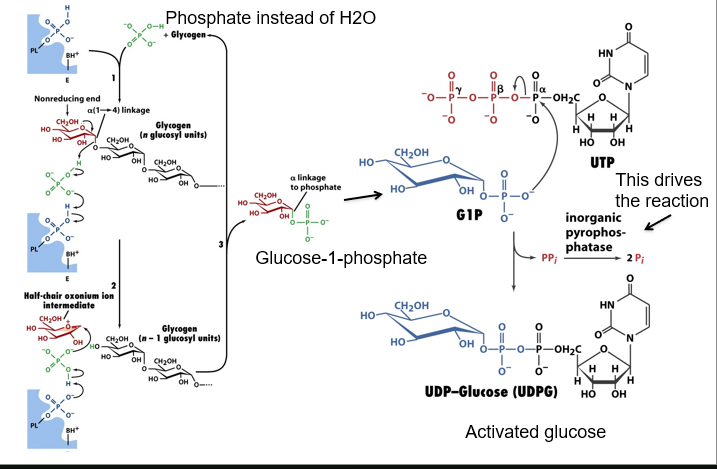
now describe how activated glucose can be used to make glycogen
UDP (good leaving group) is removed from activated glucose to make oxocarbenium (oxonium) ion intermediate via sn1
OH group from glycogen attacks oxonium, sn1 mechanism. replacement of a hydrogen from oxonium with a glycogen chain
what are glycans? describe the differences between O and N linked glycans
glycans are glycosylated proteins
O-linked is linked to serine or threonine amino acids. or lipids. while N-linked is to asparagine residues
O-linked glycans are assembled on the protein, while N-linked are pre-made in ER and then transferred to protein after
describe the glucose chemistry that occurs when heating food
monomeric sugars are released, and oxocarbenium ions can be made. this causes release of reducing sugars in biomass
why do living cells have only one reducing end in glycogen and rarely have unmodified monomeric glucose?
because free monomeric reducing sugars will have many different possible outcomes such as the maillard reaction where amino acids give amine groups to replace the C=O bond.
we want to prevent random reactions from occurring.
why is there such a range of flavors and aromas that can arise from cooking?
because cooking rleeases reducing sugars which can form all sorts of compounds, some of which add flavour and other can give aromas
how does this flashcard set’s content relate to bad health?
some maillard products are carcinogenic
similar reaction with reducing sugars occur over time in living tissue, and AGE (advanced glycation end products) accumulate
accumulation of AGE leads to ageing and links to diabetes symptoms