Chemistry Bonding-models test
1/40
Earn XP
Description and Tags
Criterion A
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
model
A representation of anything, to make it easier to understand, define, visualise or stimulate a complex concept, aspect or system. They use the most recent commonly accepted knowledge about that topic and there are different types with different specific purposes.
features of a model
Often represents something else in a way that offers insights into that something, allowing prediction to be made about how that something will behave in certain conditions.
They are very powerful tools (especially in dose-response where it connects what we are exposed to and how this might affect our health).
Crucial to understand their limitations
infrence vs evidence
Evidence refers to the data or information that directly supports the model, its the factual information that can be observed, measured, or tested. Inference refers to the logical conclusions made based on the evidence its the explanation/interpretation of the evidence.
democritus model
440 BCE: stated that everythingwas mae of tiny particles, empty space between the particles and speculated that they vary in size and shape, called them atomos in greek(indivisible).
Dalton model
1808: Challenged aristotelean theory, common substances always break down into the same elements in the same proportion and that compounds are combinations of different elements can neither be created nor destroyed.
Thompson model
1897: Discovered the electron, and created the chocolate chip/plum pudding model where an atom is positively packed matter with negatively charged particles distributed throughout.
Rutherford model
1909: He was the father of the nuclear age and he fired positively charged alpha particles at a sheet of gold and expected all particles to go through but some bounced back and deflected, concluded that atoms are mostly empty space and that most matter is concentrated at the centre(nucleus), negatively charged particles in some “cloud” around nucleus.
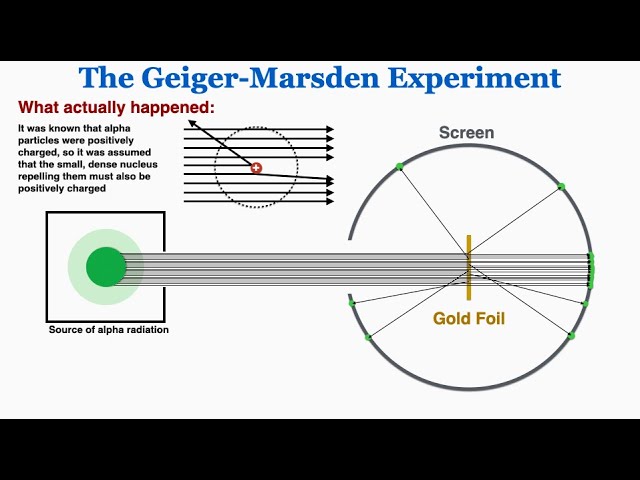
Bohr model
1913: specified that electrons orbit the nucleus at fixed energies and distances, can jump from one level to another but not be in between, planetary model.
Heisenberg model
Showed that electrons behave together like waves(not confined to one particular point in space), and that its impossible to determine the exact position and speed of electrons as they orbit , theory used in current quantum model of the atom (still being developped).
protons
Positively charged subatomic particles, weigh 1 atomic mass unit and are found at in the nucleus. Number of protons determines the element and is the atomic number on the periodic table.
neutrons
Neutral(not charged) subatomic particles that weigh 1 atomic mass unit and are foudn in int he nucleus. Number of neutrons can vary and can be found by doing mass#-atomic#.
electrons
Negatively charged subatomic particles that have no atomic mass(barely anything) and impact the reactivity of an atom. They are located in energy levels/shells and orbit aroudn the nucleus(in between empty space).
electron configuration
The outermost shell is called the valence shell, and it can contain a maximum of 8 electrons up till calcium, first shell contains 2 electrons.
hydrogen
forms cations and has one e- on last shell but also wants one more e-, but is a diatomic element, gas at room temperature.
alkali metals
They have one spare electron in the valance shell and their name derives from their vigrouous reaction with water producing hydrogen gas.
low melting and boiling points(solid at room temperature tho).
low densities
Soft, can be cut with knife, maleable
Highly reactive, increases as you go down the group
transition metals:
A collection of normal metals:
high densities and melting points compared to G1 and G2
form coloured compounds
they are brightly coloured and shiny
different and multipe oxidation states and so they can have different charges (Fe+2 or +3)
important catalysts
not very reactive and there are no trends in reactivity among them
can form more than one compound with the same element
some are magnetic
catalysts
substances that increase the rate of a chemical reaction without being used up themselves.
why do the melting and boiling points decrease as you go down the group 1 and 2 metals
Because the metal atom radius increases as there are more shells, decreasing the strength of the metallic bonds, so less energy is needed to break the bonds. Greater electron shielding(more shells protecting the electron from he positive charge). But along the period the melting point increases as the strength of the metallic bond increases needing more energy to break.
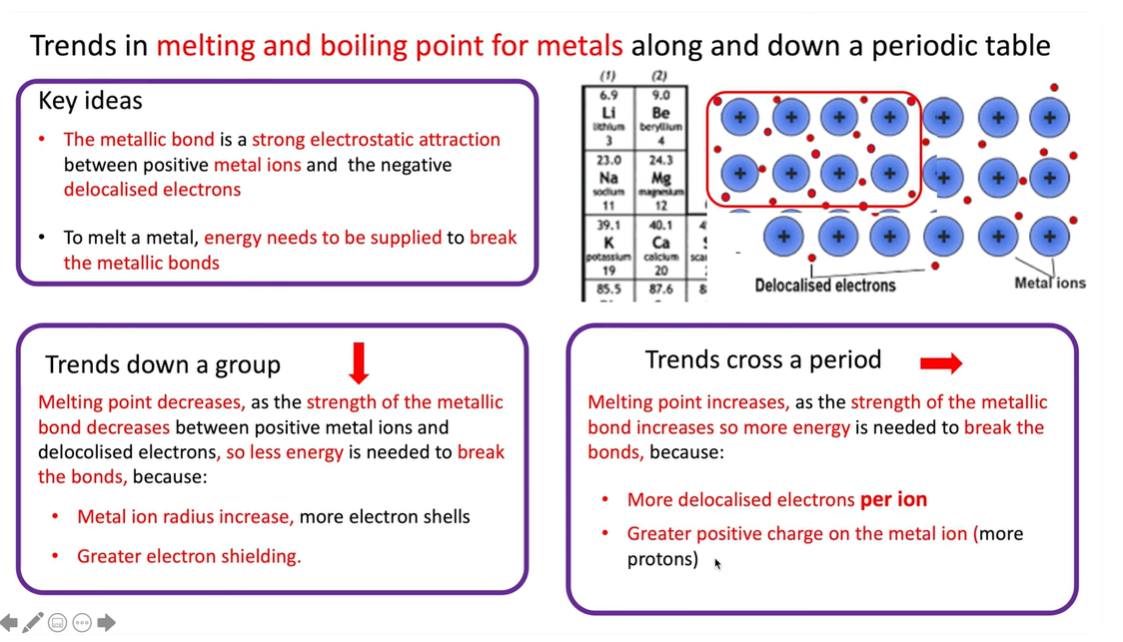
Periodic table:
The columns on the periodic table are “Groups”, and the number determines the # of electrons in valence shell. The rows on the periodic table are called “Periods” and the period # determines the # of shells the atom of a particular element has.
There are metals, semimetals and nonmetals.

State # of protons, electrons, neutrons in neautral Phosphorus atom:
Atomic #=proton #= 15
Electron number= 15
Mass #= about 40
#Neutrons= about 25
trends in reactivity for group 1 metals
The reactivity increases as you go down the group because the outer electron in the valance shell gets progressively easier to remove because the atoms get bigger as they have more shells, pushing the outer electron further away from the nucleus. This causes the attractive forces of the nucleus on the valence electron to weaken, so it can easily be lost.
halogens
Collection of diatomic non metals showing a trend in colours and state, toxic but very useful.
Often and can all exist as diatomic particles(sharing electrons)
colour intensifies as you go down the group
melting and boiling points are high and increase as you go down the group
reactivity decreases as you go down the group
State: density increases as you go down the group, F2 and Cl2 are gases, Br2 is liquid and I2 and At are solids at room temperature.
Form ionic bonds with metals(usually group 1) creating negative ions and changing the ending as they become halides.
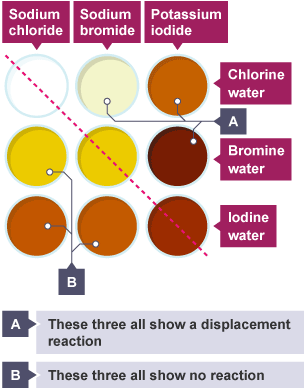
Displacement reactions can take place
Displacement reactions
The more reactive halogen displaces a less reactive halogen, stealing its bond in a compound.
write the balanced equation for:
Lithium + water = lithium hydroxide + hydrogen
2Li+2H2O=2LiOH+H2
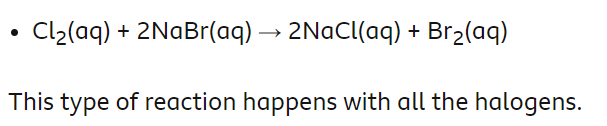
reminders for balancing equations
recognise diatomic molecules
add in "(aq, g, s, l)
can’t change subscript only coefficients
count atoms on both sides to verify
ionic bonds
the electrostatic force that holds oppositely charged ions together in a compound
Why does the reactivity decrease as you go down the group of halogens
because as you go down the group the valence shell becomes further away from the positive nucleus, so the attractive forces(needed to pull an extra electron in) become weaker, and if the halogen can’t attract the electron to fill its shell it can’t react.
why do the melting points decrease as you go down the g1 and g2 metals
It decreases as the strength of the metallic bonds decreases between the positive metal ions and delocalised electrons so less energy is needed to break the bonds:
greater metal radius, more electrons
greater electron shielding
nobel gases
Inert gases found in group 8/0 and have full valence shells making them unreactive.
They are colourless gases
exists as single attoms(monoatomic)
none flammable
boiling points increase as you go down the group
Uses of nobel gases:
ability to create inert atmospheres
Helium: fill ballons(profitable), cooling agent for MRI scanners
Argon and Krypton used for light bulbs(incandescent lamps).
Neon: Lighting, lasers and discharge tubes
Xenon: lighting for cameras, sunbed and bacterial lamps.
Isotope
Different forms of the same element but with different number of neutrons. Chemically act the same (protons and electrons control this) but vary in mass and some are radioactive whilst others are stable.
Uses of radioactive isotopes:
Medically: treatment of cancer and sterilisng medical equiptment.
Industrially: Measuring the flow in pipelines, locating leaks and measuring engine wear.
ions
An ion has unequal number of protons and electrons, making it charged overall. Thus, when two atoms react with each other electrons are either lost, gained or shared as all atoms want to have full shells. You add a +x or a -x on top of abbreviation.
coins
metal features making htem durable yet maleable so they can be moulded into shape
Electrical wires
good electrical and thermal conductors and ductile
building bridges and large structures
high density, strong an dhard and hgih tencile strength
pots and pans
have high melting and boiling points
makeup and paints
they form coloured compounfs which can colour pigment.
transporting oxygen in red blood cells
iron acts as binding sites for oxygen in hemaoglobin and forms coloured compounfs and so it cna be used to create/show colour of our blood relative to its condition.
uses of halogens
fluorine is used as a fluoride in toothpaste because it helps strengthen and protect the teeth from decay as it is effective in killing bacteria and can react with calcium.
chlorine is used in bleach as it oxidises so it can break down chemical bonds present in pigments/dyes, removing colour.
bromine is used as a fire retardant because it can slow down combustion reactions and has a high melting and boiling point so it can stay stable at high temperatures.
uses of alkali metals
lithium is used in batteries because its light yet charged and reactive, its also a relatively good conductor of electricity.