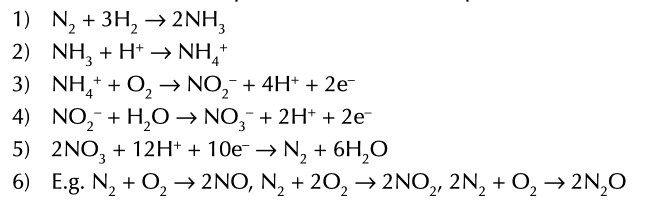6. chemical industry
5.0(1)
5.0(1)
Card Sorting
1/74
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
75 Terms
1
New cards
rate of reaction
change in amount of reactants or products per unit time
2
New cards
continuous monitoring
a way of measuring rate of reaction over the complete course of the reaction
3
New cards
methods of measuring rate of reaction
chosen based on what changes in the reaction:
* pH measurement
* gas volume
* loss of mass
* colour change
* titration
* pH measurement
* gas volume
* loss of mass
* colour change
* titration
4
New cards
pH measurement
used if a product as an acid or base, can be measured with pH meter or a pH probe with a data logger, can be converted to concentration by \[H+\] = 10^-pH, gives mol dm-3 time -1
5
New cards
gas volume
if produced it can be collected in a gas syringe and volume recorded at regular intervals, gives volume time-1
6
New cards
loss of mass
measure mass remaining of a system if a gas is given off, measured with a balance, gives mass time-1
7
New cards
colour change
if a reactant or product is coloured, the colour change of the reaction can be tracked with a colorimeter measuring absorbance (more concentrated means more absorbance)calculate concentration with calibration curve
8
New cards
titration
small sample are taken at intervals and titrated, the samples are slowed down by diluting with deionised water, cooling or stoped with a chemical
9
New cards
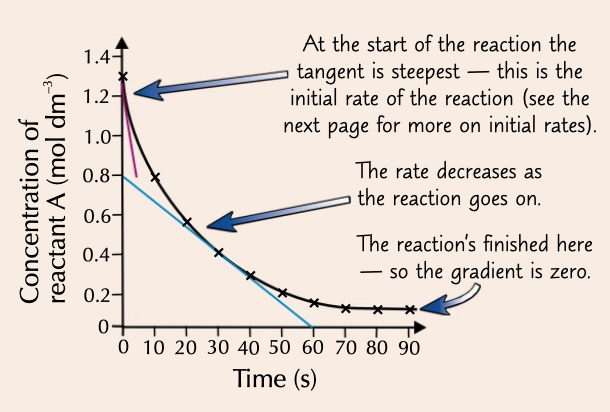
concentration time graph
a graph that can be used to work out rate of reaction by determining gradient at any point with a tangent
10
New cards
initial rate
the rate of reaction at the start
= amount of reactant used or product formed ÷ time
= amount of reactant used or product formed ÷ time
11
New cards
initial rate method
the time for a set amount of product to form (eg gas) is recorded and used to calculate initial rate,
repeated serval times and the initial concentration of a reactant is changed
repeated serval times and the initial concentration of a reactant is changed
12
New cards
assumptions of initial rate method
* concentration of other reactants isn’t changing much- typically done by having them in excess
* temperature stays constant
* reactant hasn’t proceeded to far when measurement is taken
* temperature stays constant
* reactant hasn’t proceeded to far when measurement is taken
13
New cards
clock reaction
a initial rates reaction where you measure time taken for a set amount of product to form and concentration of a reactant is changed, but has an early observable end point like a colour change, quicker colour change means faster rate of reaction
14
New cards
iodine clock reaction
* small amount of sodium thiosulfate solution and starch are added to an excess of hydrogen peroxide and iodine ions in acid solution
* when the sodium thiosulfate is added it reacts instantaneously with any iodine that forms
* initially all iodine made is used up immediately, but once sodium thiosulfate is used any more iodine stays in solution storing starch blue black (starch turns blue back in presence of iodine so acts as an indicator), this is the end point
* changing the concentration of iodine ions or hydrogen peroxide will give different times for the colour change
* when the sodium thiosulfate is added it reacts instantaneously with any iodine that forms
* initially all iodine made is used up immediately, but once sodium thiosulfate is used any more iodine stays in solution storing starch blue black (starch turns blue back in presence of iodine so acts as an indicator), this is the end point
* changing the concentration of iodine ions or hydrogen peroxide will give different times for the colour change
15
New cards
rate equation
tells you how rate is affected by concentration of reactants
rate = k\[A\]m\[B\]n
rate = k\[A\]m\[B\]n
16
New cards
rate constant
k
bigger it is the water the reaction
remains the same for a reaction at a particulate temperature
unit vary
= rate ÷ \[A\]m\[B\]n
bigger it is the water the reaction
remains the same for a reaction at a particulate temperature
unit vary
= rate ÷ \[A\]m\[B\]n
17
New cards
orders
tell you how a reactants concentration affects the rate
determined only by reactions
determined only by reactions
18
New cards
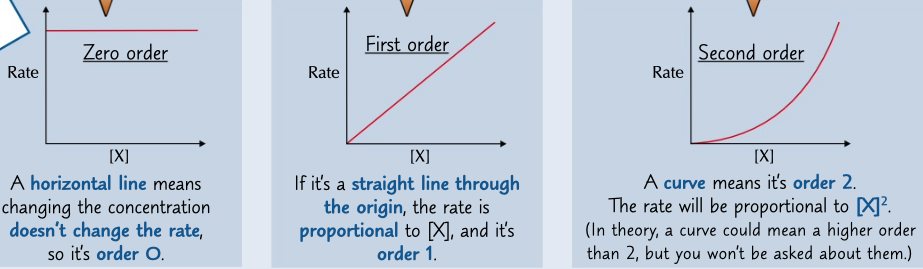
zero order
order if concentration of the reactant changes and rate does not
19
New cards
first order
order of concentration of the reactant changes and the rate changes proportionally
eg if conc doubles then rate doubles
eg if conc doubles then rate doubles
20
New cards
second order
oder if concentration of reactant changes and rate changes proportionally to \[A\]^2
eg if conc doubles then rate will be 2^2 so 4 times faster
eg if conc doubles then rate will be 2^2 so 4 times faster
21
New cards
overall order
sum of individual orders of reactants, gives order of reaction
22
New cards
determine order
by:
* constructing a rate concertation graph
* comparing initial rate for different concentration
* constructing a conception time graph and comparing half lives
* constructing a rate concertation graph
* comparing initial rate for different concentration
* constructing a conception time graph and comparing half lives
23
New cards
rate concentration graph
made by constructing a concentration time graph and calculating rate at various concentrations and plotting it on a new graph
24
New cards
units of rate constant
calculated by substituting units for rate and concentration in to rate equation
done by cancelling out units
eg units of k = mol dm-3 s-1 ÷ (mol dm-3)2 (mol dm-3) = mol-2 dm6 s-1
done by cancelling out units
eg units of k = mol dm-3 s-1 ÷ (mol dm-3)2 (mol dm-3) = mol-2 dm6 s-1
25
New cards
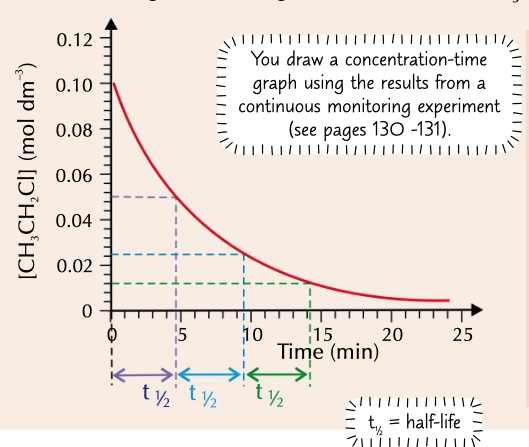
half life
the time taken for a reactant to halve in quantity (half to be used up)
easily calculated on concentration time graph
easily calculated on concentration time graph
26
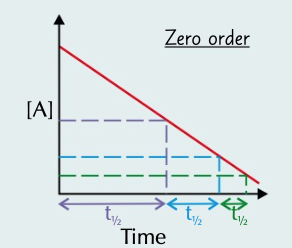
New cards
half life increases
half life of a zero order reaction where the rate doesn’t change
27
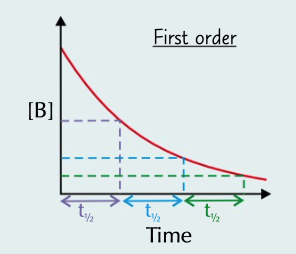
New cards
half life is the same
half life of a first order reaction where rate is proportional
28
New cards
half life increases
half life of a second order reaction where rate is proportion to x2
29
New cards
word out rate constant from half life
using equation k = ln2 ÷ half life time
units for each are: no units ÷ s = s-1
works for first order reaction with equal half life
units for each are: no units ÷ s = s-1
works for first order reaction with equal half life

30
New cards
temperature
effects rate of reaction as:
* increases collisions
* increases reactants with activation energy
* more reactants have the right orientation
* more kinetic energy
* increases collisions
* increases reactants with activation energy
* more reactants have the right orientation
* more kinetic energy
31
New cards
higher temperature
higher rate constant
higher rate of reaction
higher rate of reaction
32
New cards
Arrhenius equation
links rate constant and activation energy and temperature
k = Ae^-Ea/RT given on data sheet
Ea = activation energy J mol-1
T = temperature K
R = gas constant 8.31 J K-1 mol-1
A = pre-exponetial factor (a constant)
e = exponential relationship (button on calculator)
k = Ae^-Ea/RT given on data sheet
Ea = activation energy J mol-1
T = temperature K
R = gas constant 8.31 J K-1 mol-1
A = pre-exponetial factor (a constant)
e = exponential relationship (button on calculator)
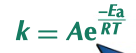
33
New cards
activation energy
as it gets bigger, k gets smaller
34
New cards
high activation energy
means there will be a slow rate and k will be smaller
35
New cards
high temperature
as it increases, k rises as more reactants have the reaction energy
36
New cards
logarithmic form
allows Arrhenius equation to calculate activation energy
ln k = -(Ea/RT) = ln A
ln = logarithmic button
ln k = -(Ea/RT) = ln A
ln = logarithmic button

37
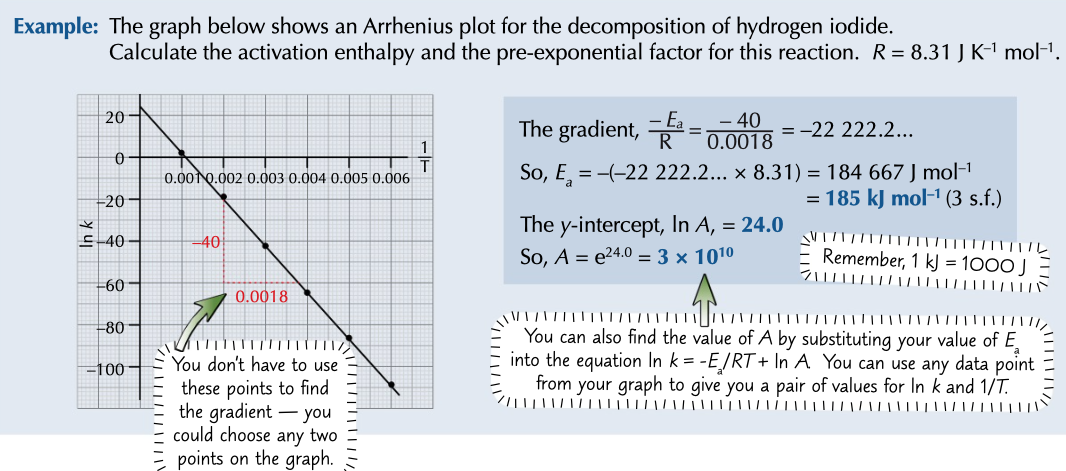
New cards
Arrhenius plot
made by plotting ln k again 1/T
produces a graph with a gradient of -Ea / R and a y-intercept of ln A
used to find activation energy and pre-exponetial factor (A)
work out gradient then times it by R (8.31 J K-1 mol-1) to give activation energy
find y intercept and use e, this works out A, or substitute in values to equation
produces a graph with a gradient of -Ea / R and a y-intercept of ln A
used to find activation energy and pre-exponetial factor (A)
work out gradient then times it by R (8.31 J K-1 mol-1) to give activation energy
find y intercept and use e, this works out A, or substitute in values to equation
38
New cards
rate determining step
slowest step in a multistep reaction
at least one reactant must appear in rate equation
used to predict rate determining step
at least one reactant must appear in rate equation
used to predict rate determining step
39
New cards
affect the rate
reactants that appear on rate equation
40
New cards
order of a reactant
tells you how many molecules of that reactant are involved in rate determining step
41
New cards
mechanism
can be predicted with the rate equation, based on what appears in rate determining step
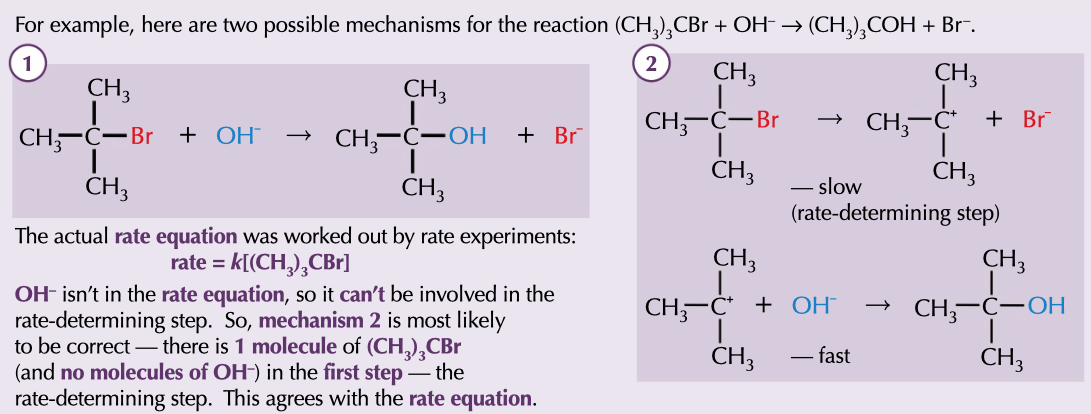
42
New cards
decomposition
can mean that there can be one reactant with 2 moles in rate determining step but its not second order as it breaks down by itself instead of reacting together
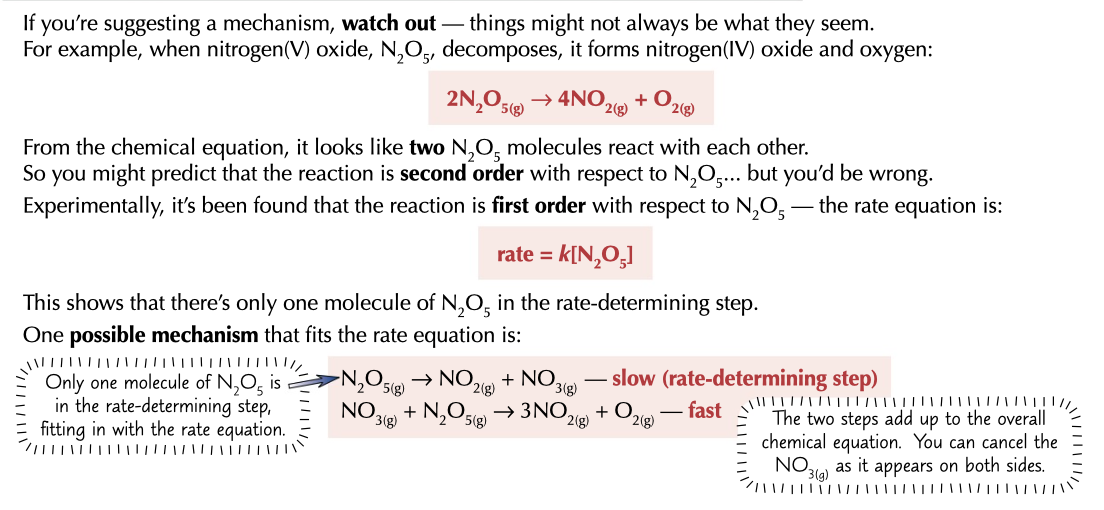
43
New cards
costs involved in producing a chemical
raw materials
fuel/energy
overheads/fixed costs
disposal costs
fuel/energy
overheads/fixed costs
disposal costs
44
New cards
raw materials
chemicals needed for the reaction
cheap, widely available ones are best
cheap, widely available ones are best
45
New cards
fuel/energy
reactions needing Hugh temperature or pressure use more energy
transporting chemicals uses energy
transporting chemicals uses energy
46
New cards
overheads/fixes costs
costs that need to be met regularly, including staff wages, rent, taxes, insurance, bills, etc
47
New cards
disposal costs
any unwanted by-products need to be disposed of safely
48
New cards
temperature
high means faster rate of reaction by more expensive
49
New cards
pressure
higher makes gaseous reaction faster but uses lots of energy so expensive, also very dangerous
50
New cards
catalyst
used to speed up reactions allowing for lower temperatures, but can be expensive and may have to be separated form products if in same state
good investment as don’t get used up
good investment as don’t get used up
51
New cards
equilibrium constant
ratio of products and reactants at dynamic equilibrium , represented by Kc
Kc = \[D\]d\[E\]e / \[A\]a\[B\]b
can also be used to determine concentration of things given Kc and some concentrations
Kc = \[D\]d\[E\]e / \[A\]a\[B\]b
can also be used to determine concentration of things given Kc and some concentrations
![ratio of products and reactants at dynamic equilibrium , represented by Kc
Kc = \[D\]d\[E\]e / \[A\]a\[B\]b
can also be used to determine concentration of things given Kc and some concentrations](https://knowt-user-attachments.s3.amazonaws.com/c3b3a633ce1d43ba84bcec562c7ebc24.jpeg)
52
New cards
dynamic equilibrium
when the forwards and backwards reaction cancel each other out in a reversible reaction
53
New cards
alter Kc
temperature changes do but pressure changes do not
54
New cards
effect of temperature on Kc
if increased reaction shifts endothermic direction
if decreased reaction shifts to exothermic direction
if more product formed then Kc rises and the opposite the other way round
if decreased reaction shifts to exothermic direction
if more product formed then Kc rises and the opposite the other way round
55
New cards
effect of pressure on Kc
as the reaction always reverses the change there is not effect on Kc
56
New cards
effect of catalysts on Kc
the have no effect as they do not increase yield they only mean equilibrium is approached faster
57
New cards
how economical a reaction is
determined by position of equilibrium and the yield of the reaction
generally a compromise of conditions
generally a compromise of conditions
58
New cards
harder process
carried out at 400˚c and 200 atmospheres of pressure
higher pressure makes it faster but very expensive
high temperature increases rate but decreases yield as forward reaction is exothermic so equilibrium gets shifted to left, so compromise between speed and yield
higher pressure makes it faster but very expensive
high temperature increases rate but decreases yield as forward reaction is exothermic so equilibrium gets shifted to left, so compromise between speed and yield
59
New cards
risks of production
some chemicals are high flammable and explosive eg pressured hydrogen
some chemicals are toxic/harmful to health eg chlorine
some chemicals damage the environment eg acid rain
some chemicals are toxic/harmful to health eg chlorine
some chemicals damage the environment eg acid rain
60
New cards
Kc units
put the units into Kc equation and cancel them out
(mol dm-3) (mol dm-3) / (mol dm-3) = mol dm-3
(mol dm-3) (mol dm-3) / (mol dm-3) = mol dm-3
61
New cards
find concentrations at equilibrium
by using a technique that won’t disturb position of the equilibrium eg colorimetry or pH
62
New cards
colorimetry
can be used to determine concentrations of a reaction if it is coloured, find absorbance and use a calibration curve to determine concentration
63
New cards
pH
if one side of the reversible reaction contains an acid or base at equilibrium the a pH probe can be used and concentration calculated
64
New cards
never use titration
as it would cause any reactants/products to to react and from a salt and water decreasing the concentration therefore shifting the equilibrium, this means it cannot be used to find equilibrium concentrations
65
New cards
ice tables
method to work out initial concentrations and equilibrium concentrations
initial eg 1
change eg -0.5
equilibrium eg 0.5
initial eg 1
change eg -0.5
equilibrium eg 0.5
66
New cards
diatomic molecule
nitrogen, top of group 5, electronic configuration of 1s2 2s2 2p3 so there’s 5 electrons inter shell they pair up to share 3 of them making a triple bond, difficult to break so there unreactive
67
New cards
ammonia NH3
formed form 1 N and 3H, forms 3 covalent bonds and has one lone pair
can form hydrogen bonds so is very soluble in water
acts as a by the lone pair forming a dative covalent bond with complex ions with transition metals
lone pair also causes It to act as a base as it can from date covalent bond with H+
can form hydrogen bonds so is very soluble in water
acts as a by the lone pair forming a dative covalent bond with complex ions with transition metals
lone pair also causes It to act as a base as it can from date covalent bond with H+
68
New cards
nitrogen oxides
NO, N2O and NO2
69
New cards
NO
nitrogen monoxide, nitric oxide, or nitrogen (ii) oxide, colourless gas
70
New cards
N2O
dinitrogen monoxide, nitrous oxide or nitrogen (i) oxide (laughing gas) sweet smell and colourless
71
New cards
NO2
nitrogen dioxide or notion (IV) oxide, down gas, sharp order and is toxic
72
New cards
sodium hydroxide
added to a mixture to test for NH4+ (ammonium ions), gently heated, causes ammonia gas to be produced which is alkaline so turns damp red litmus paper blue
73
New cards
aluminium
used to test for NO3- (nitrate (V) ions) by warming solution with sodium hydroxide solution (devardas alloy also used, contains aluminium), the nitrate ions are reduced in the presence of the alkali, ammonia gas is produced, turns damp red litmus paper blue
74
New cards
nitrogen cycle
process where nitrogen goes to ammonia, ammonium ions, nitrate (III) ions, nitrate (V) ions and back to nitrogen to to nitrogen oxides
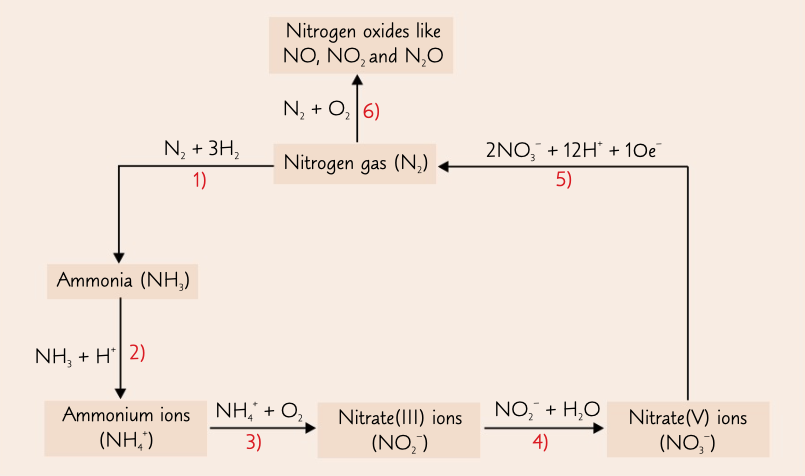
75
New cards
reduce half equations
involved in nitrogen cycle