arenes
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
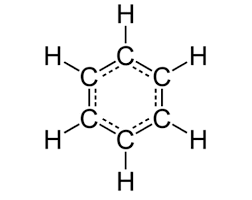
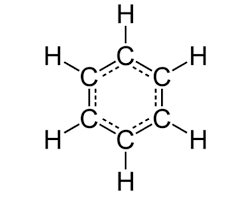
benzene
-cyclic, planar to achieve maximum overlap
-C6H6
-C bonded to 2 other Cs and one H by sigma bonds, leaving a lone e- in p-orbital (sits above and below planar ring)
-p-orbitals overlap sideways to form pi system
-delocalised e- ring
-high e- density above and below ring
-very stable
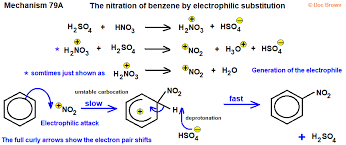
electrophilic substitution
-generation of electrophile (requires catalyst)
-electrophile attached to benzene to form intermediate
-H+ lost from intermediate, reforming delocalised ring
NO2 replaces H
-regeneration of catalayst
activating and deactivating groups
-OH and NH2 (and CH3) are activating, they stabilize substitutions at 2,4 positions, electron donating groups
-NO2 and Cl deactivate ring by attracting e- out of benzene ring, electron withdrawing, so less likely for multiple substitutions, direct at 3,5 positions
thermochemical evidence for benzene’s structure
-thermochemical: enthalpy change of hydrogenation was predicted to be -360kJ, 3x enthalpy change of cyclohexene
-it was actually -208kJ (less negative/exothermic than expected) so more stable than cyclohexatriene
x-ray diffraction to prove benzene model
-every benzene C-C bond length in intermediate length between double and single bond, so can’t be cyclohexatriene
reaction with bromine to prove benzene structure
-doesn’t decolourise without catalsyt, so no double bonds
halogenation with bromine and chlorine
-Br2 + FeBr3 (catalyst/halogen carrier) => Br+ + FeBr4-
-Cl2 + AlCl3 => Cl+ + AlCl4-
-FeBr3 + HBr OR AlCl3 + HCl are regenerated as hydrogen lost from intermediate
nitration
-conditions: 50 degrees C with conc. HNO3 and H2SO4
-HNO3 + H2SO4 => H2NO3+ + HSO4- (H+ transferred)
-H2NO3+ => H2O + NO2+ (electrophile)
-regeneration of H2SO4: H+ + HSO4-
alkylation
-conditions: room temp., AlCl3 catalyst, haloalkane
-CH3Cl + AlCl3 = AlCl4- + CH3+ (alkyl group)
-multiple substitutions
acylation
-conditions: 60 degrees C, reflux, AlCl3 catalyst, anhydrous
-monosubstitution
-RCOCl (acyl chloride) + AlCl3 (halogen carrier) = AlCl4- + RCO+ (electrophile)
-regeneration of AlCl3: H+ + AlCl4-
why is benzene more resistant to bromination compared to alkenes
In benzene pi e- are delocalised (but localised in C=C),
-it has a lower e- density so polarises br2 less
it’s more stable compared to localised e- density of pi bond in alkenes
why are multiple substitutions for alkylation possible…
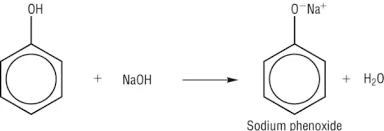
phenols
-not alcohols
-white crystalline solids at room temp.
-C6H5OH
-weak acid (so only react with strong bases e.g. NaOH, not NaCO3)
-kills bacteria (disinfectant) but corrosive to skin
-burns a smoky flame due to high ration of C atoms and incomplete combustion
-sparingly soluble, non-polar benzene ring disrupts formation of hydrogen bonds
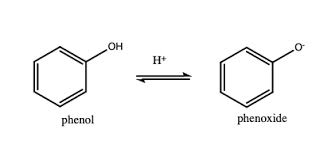
-can lose H+ as phenoxide ion is stabilised, -ve charge on oxygen is delocalised around ring, overlaps with delocalised e- ring
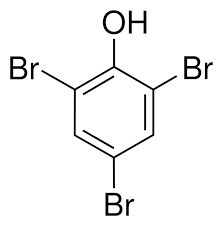
Bromination of phenol
-triple substitution with bromine water and room temp.
-forms white ppt (test for phenols), 2,4,6- tribromophenol (C6H2Br3OH)
And Hbr
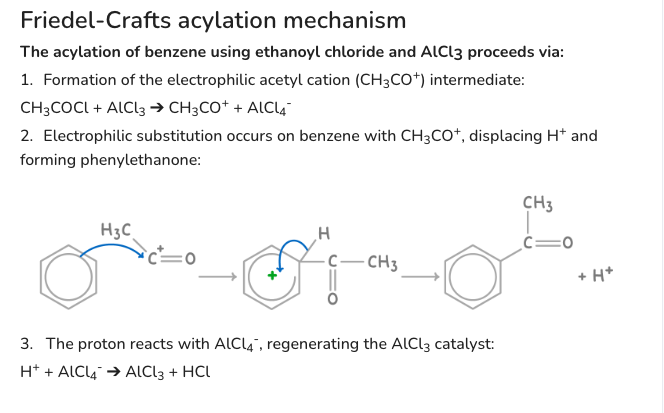
Why is phenol more reactive than benzene
(OH activates e- ring)
-lone e- pair from oxygen’s p-orbital is delocalised/donated into pi ring
-increasing e- density in ring
-so polarises br-br, generating electrophile br+, more susceptible to electrophilic attack
- (halogen carrier isn’t needed)
Phenol + dilute nitric acid
2 or 4 nitrophenol
doesn’t require conc. H2SO4 catalyst or HNO3 to be conc. as phenol more reactive tha benzene
phenols in alkalis/strong bases
-soluble in sodium hydroxide as phenoxide ions attract water molecules, making ionic-dipole interactions, stronger than hydrogen bonds