Module 12 Online HW Review Bonus
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
What is the total number of electrons in the correct Lewis dot formula of the SO3- ion?
26
3 multiple choice options
Which of the following is a correct Lewis structure for oxygen?
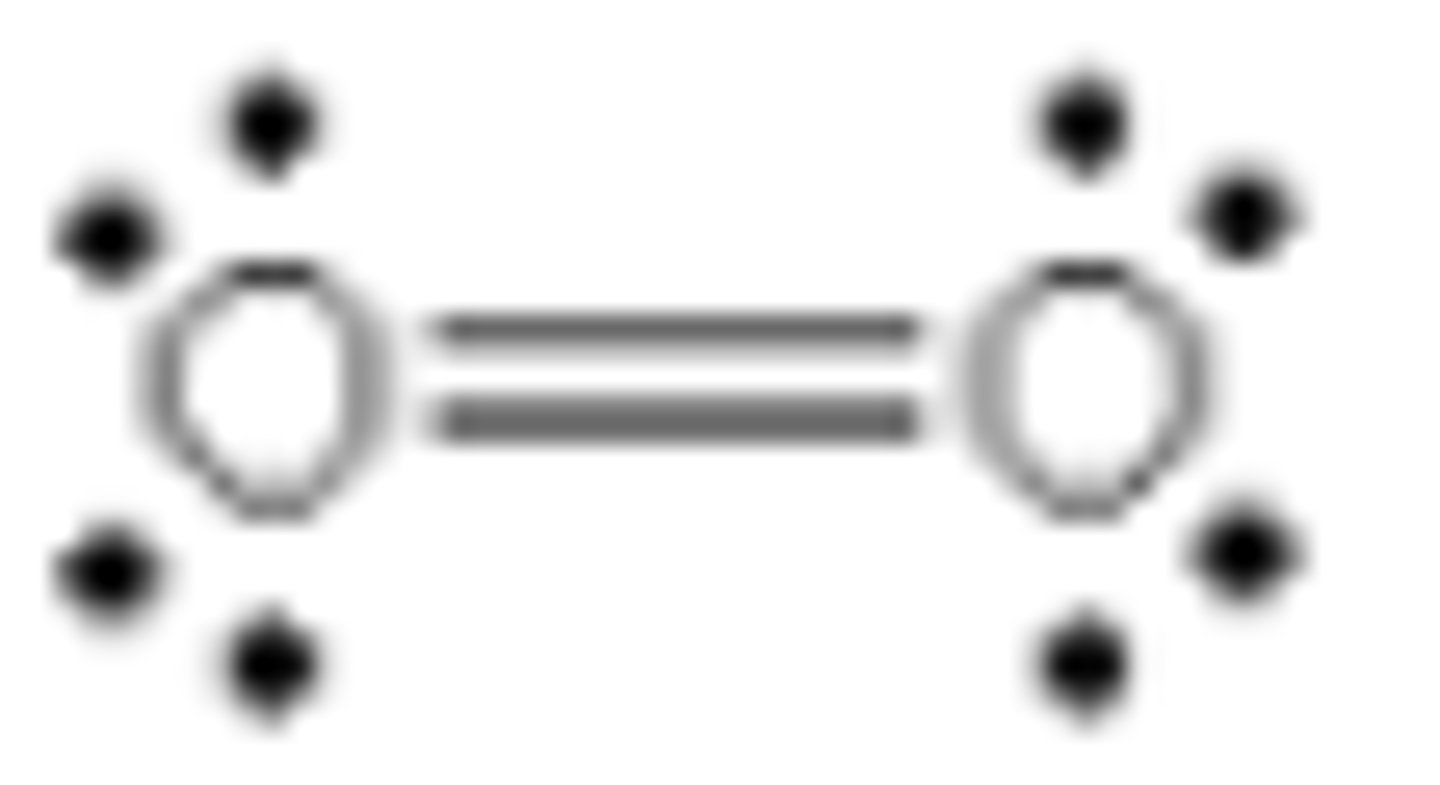
Of the following elements, which has the lowest electronegativity?
Ca
3 multiple choice options
Which of the following pairs of elements and valence electrons is incorrect?
S - 4
3 multiple choice options
In the best Lewis structure for NO+, what is the formal charge on the N atom?
0
3 multiple choice options
Consider the bicarbonate ion (also called the hydrogen carbonate ion). After drawing the correct Lewis dot structure(s), you would see:
two equivalent resonance forms.
3 multiple choice options
The central atom in BF3 has __ bonding pairs of electrons and __ non-bonding pairs of electrons.
3, 0
1 multiple choice option
Which one of the following violates the octet rule?
AsF5
3 multiple choice options
A Lewis dot structure for this atom has five electrons around the symbol. Which of the following could be the atom?
phosphorous
3 multiple choice options
Which of the following species have the same molecular geometry: CO2, H2O, BeCl2, and N2O?
CO2, BeCl2, and N2O
3 multiple choice options
When melting S8, ________ forces must be overcome and S8 is expected to have a ________ melting point than MgS.
intermolecular, lower
3 multiple choice options
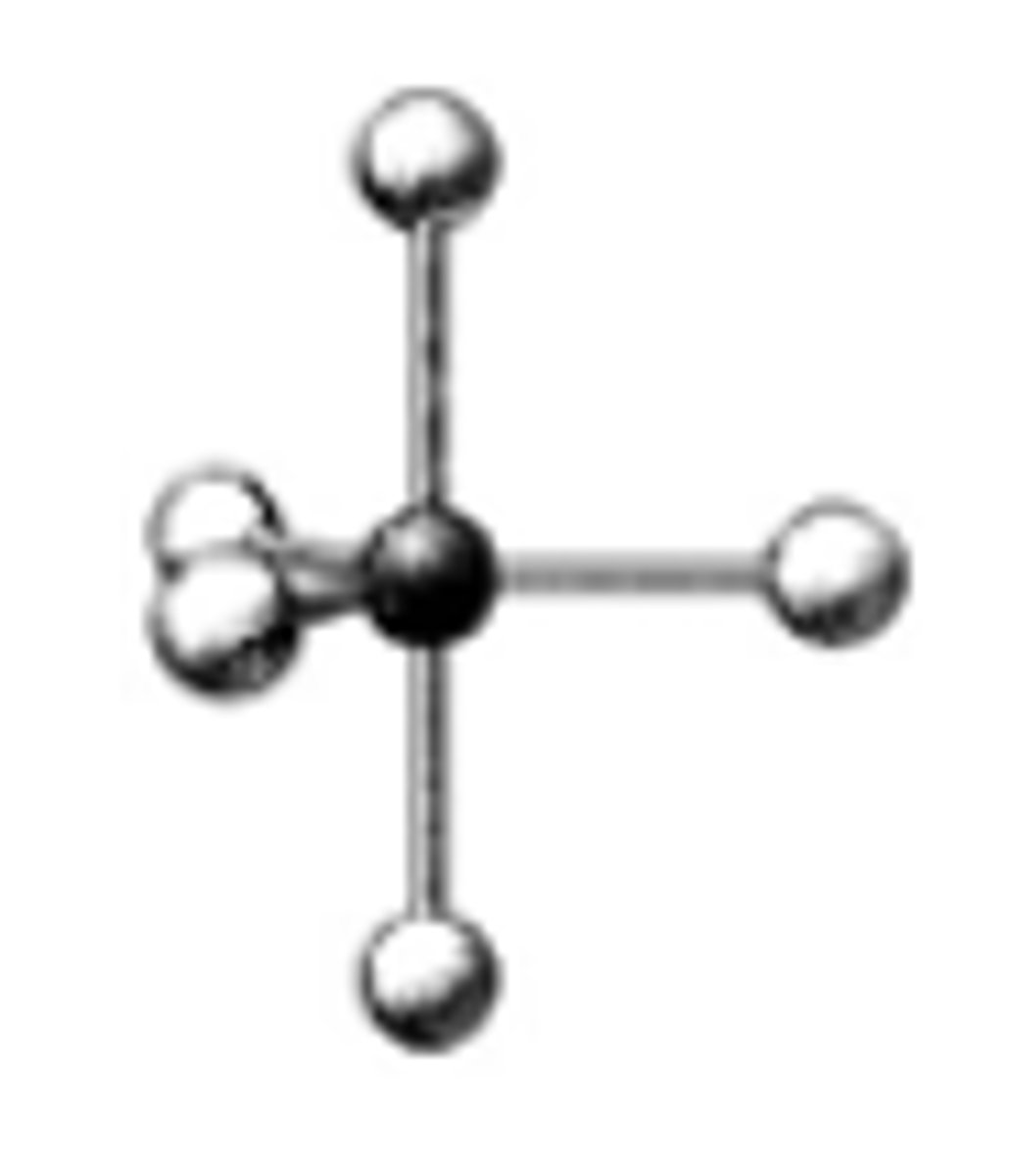
What are the bond angles in the following molecular model of PF5?
90°, 120°, and 180°
3 multiple choice options
Which of the following molecules does not have a net dipole moment?
BH3
3 multiple choice options
What is the electronic geometry of the central atom of sulfur dioxide?
trigonal planar
3 multiple choice options
Which molecule has the weakest bonds?
CI4
3 multiple choice options
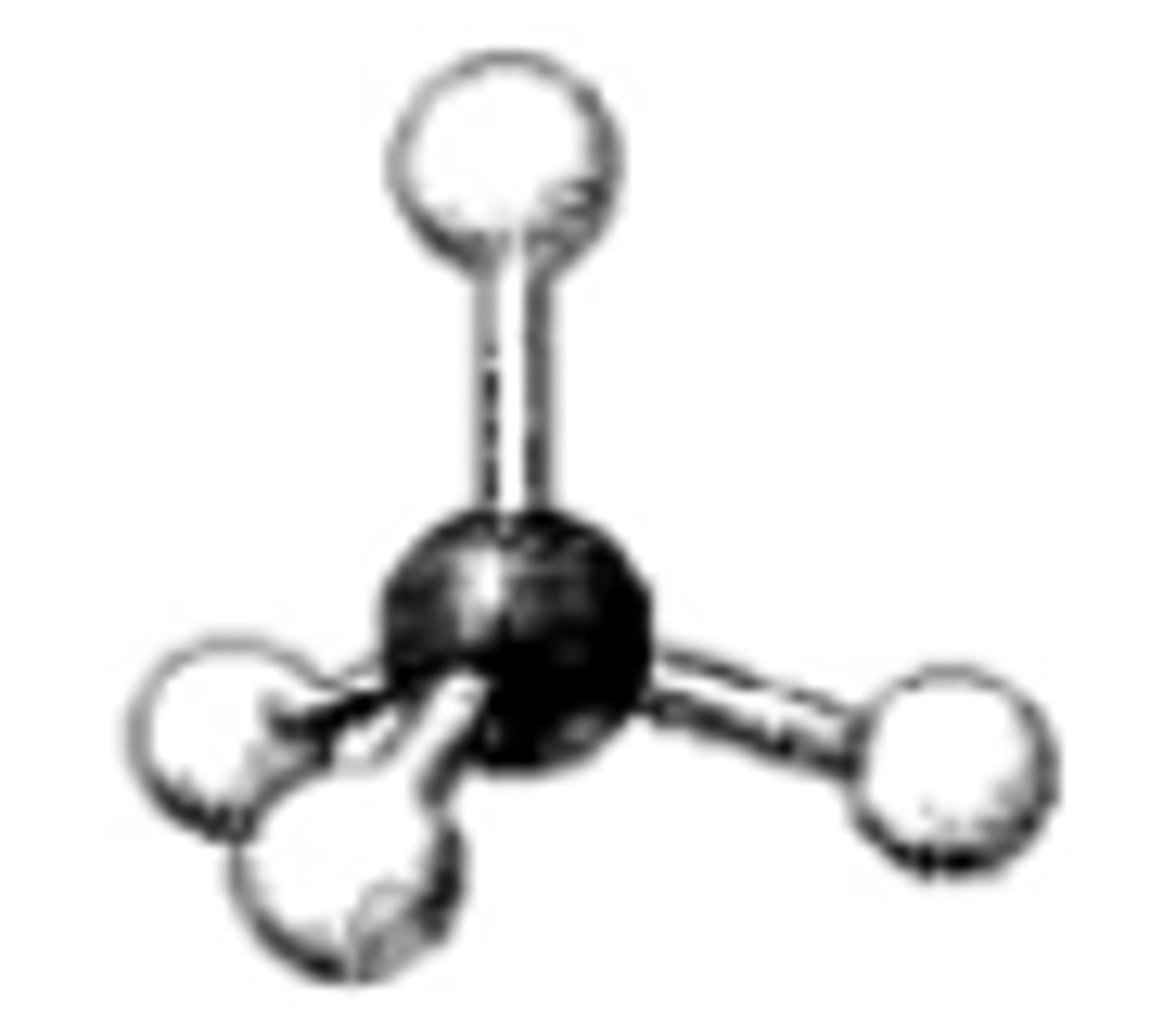
What is the geometry around the central atom in the following molecular model of NH4+?
tetrahedral
3 multiple choice options
Which is the longest bond?
N-N
3 multiple choice options
Which one of the compounds below is most likely to be ionic?
ScCl3
3 multiple choice options
In the Lewis structure for the OF2 molecule, the number of lone pairs of electrons around the central oxygen atom is:
2
3 multiple choice options
An example of a molecular compound that obeys the octet rule in which all atoms have a zero formal charge is:
NCl3
3 multiple choice options
Which drawing best shows the direction of the dipole moment in H2C=CCl2?
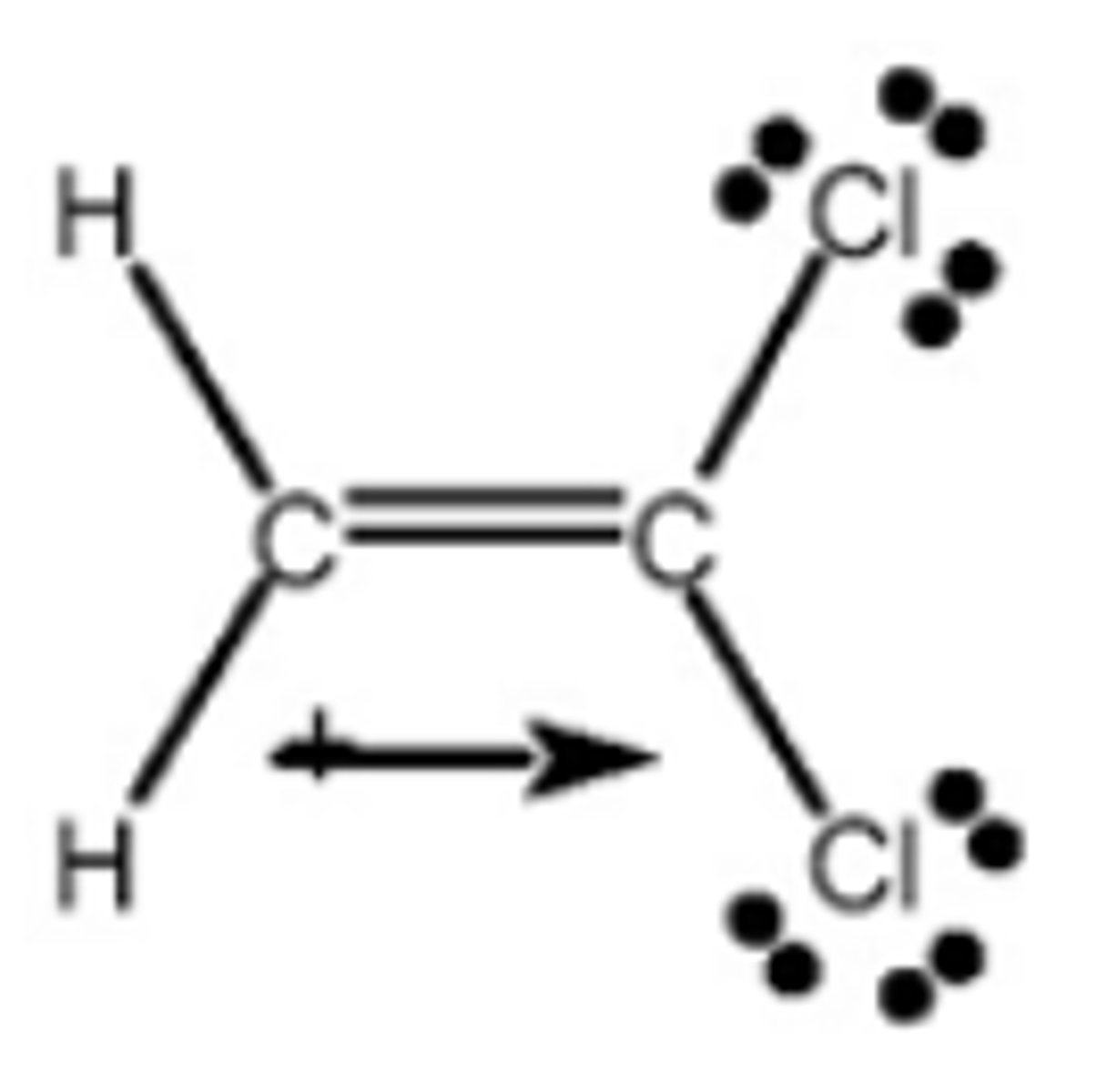
Which structure(s) contain(s) an oxygen atom that bears a formal charge of +1?
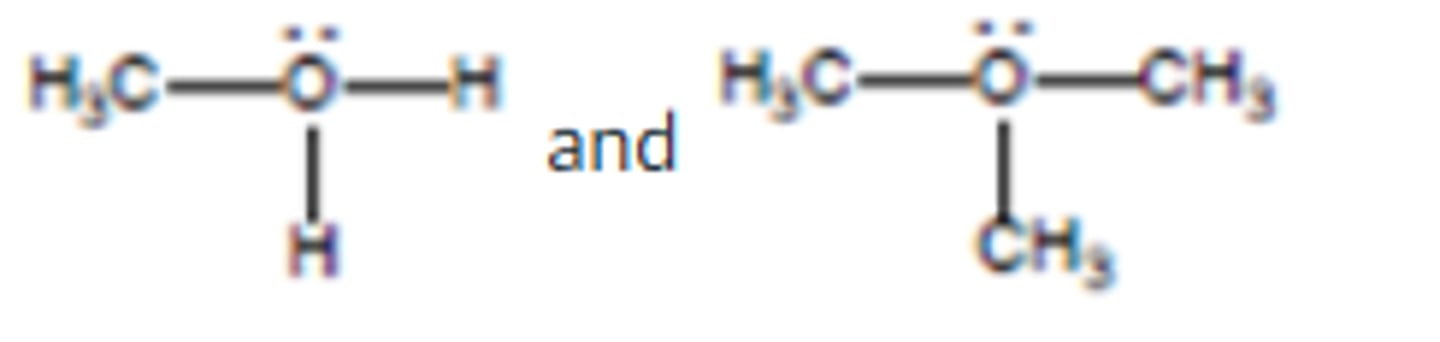
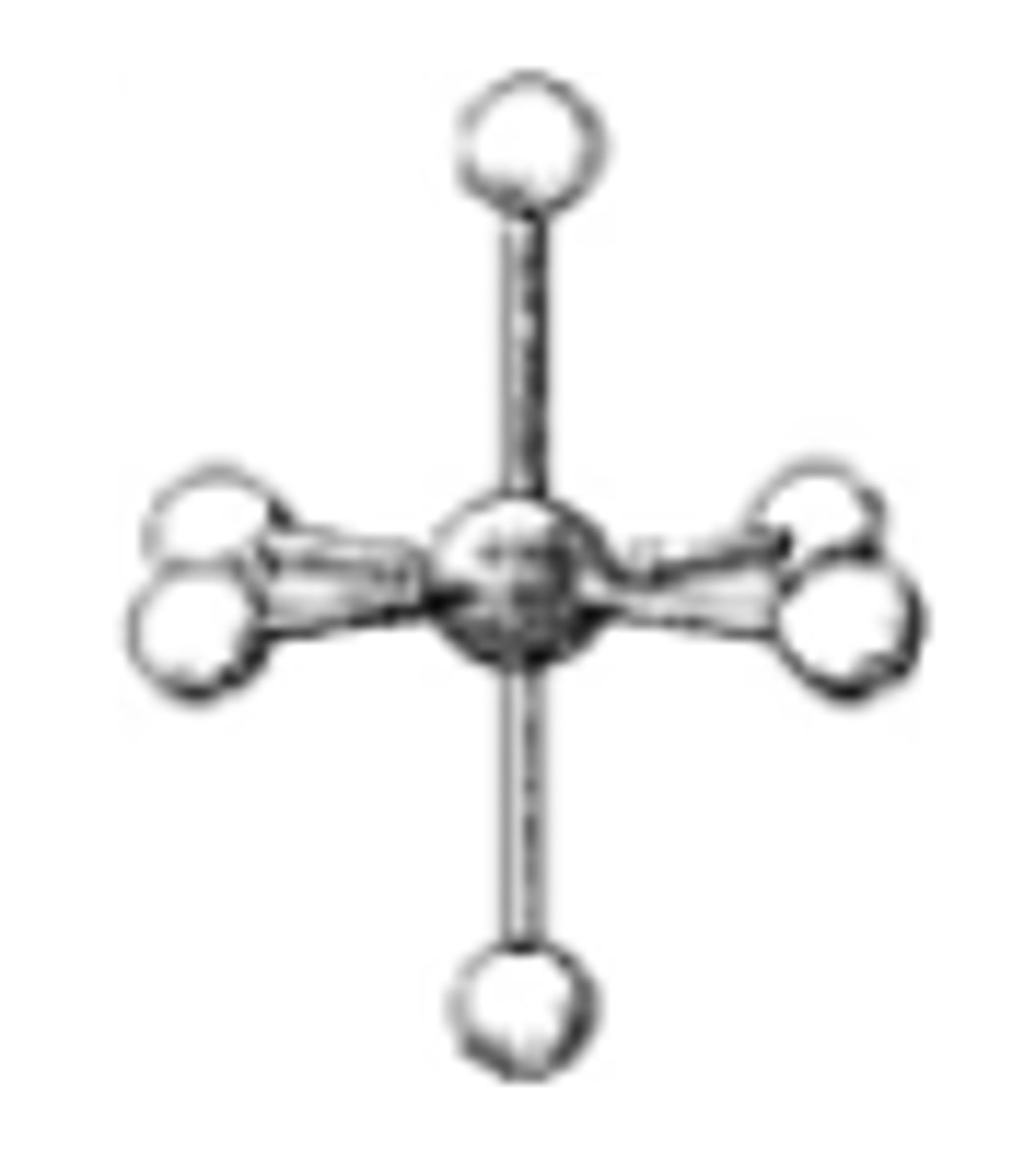
What is the geometry around the central atom in the following molecular model of SF6?
octahedral
3 multiple choice options
A chlorine atom in Cl2 should have a:
charge of 0.
3 multiple choice options
The correct dot formulation for nitrogen trichloride has:
3 N-Cl bonds and 10 lone pairs of electrons.
3 multiple choice options
The valence electrons of representative elements are:
in the outermost occupied major energy level.
3 multiple choice options
The correct electron-dot formulation for HCN shows:
1 C≡N, 1 C-H bond, and 1 lone pair on the N atom.
3 multiple choice options
What is the hybridization of the central atom is BeBr2?
sp
3 multiple choice options
Based on VSEPR theory, which should have the smallest XAX bond angle?

What is the electronic geometry of the central atom of carbon dioxide?
linear
3 multiple choice options