Renal Transport of Bicarbonate and Hydrogen Ion Transport
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
What are the two big challenges to maintaining a pH of 7.4? How is this handled in our body?
Plasma pH of 7.4 is challenged constantly by metabolic and dietary activity
1) Aerobic Metabolism
→ produces large amounts of CO2 which would cause a pronounced drop in pH
2) Anaerobic Metabolism
→ produces a small amount of things like lactate which can not be blown off by the lungs
→ causes a drop in plasma pH
3) Dietary
→ we eat acids and lose some base in our feces in a day
This extra accumulated acid needs to be excreted by our kidneys (around 70mmole) and needs to reabsorb bicarbonate
What are the three systems that control plasma pH?
1) Buffers - anything that minimizes a change in pH with the most important one being the CO2-bicarbonate buffer
→ because our lungs are an open system and there are large amounts of bicarbonate this system is effective even with a relatively low pKa
2) Lungs - regulates the CO2 levels of the bicarbonate buffering system
3) Kidneys - regulates bicarbonate levels and excretes nonvolatile acids
→ kidneys will secrete protons into the tubule lumen which aids in reabsorption of bicarbonate and excretion of nonvolatile acid
What is Net Urinary Acid Excretion
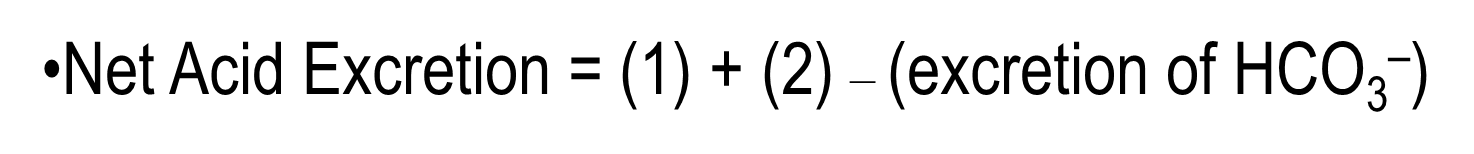
Net Urinary Acid Excretion involves protons that are excreted being bound to buffers minus the excretion of bicarbonate
1) Filtered buffers such as phosphate can bind to protons and create titratable acids
2) Protons can also be excreted in the form of Ammonium or NH4+ in the urine
The final equation then is: 1+2 - (excretion of bicarbonate)
How does our body handle an acid challenge
Our body handles an acid challenge in three steps:
1) Following an increase in acid, plasma bicarbonate and non-bicarbonate buffers will neutralize the majority of the hydrogen introduced
→ bicarbonate + Hydrogen will be converted to water and CO2
→ nonbicarbonate buffers will combine with protons as well
2) Generated CO2 created by the bicarbonate buffer system is excreted by the lungs
3) Kidneys generate new bicarbonate in order to replace the consumed bicarbonate in step 1
→ Bicarbonate will also be used to strip off the proton from the non-bicarbonate buffers created in step 1 as well
What is the process of hydrogen secretion and bicarbonate reabsorption?
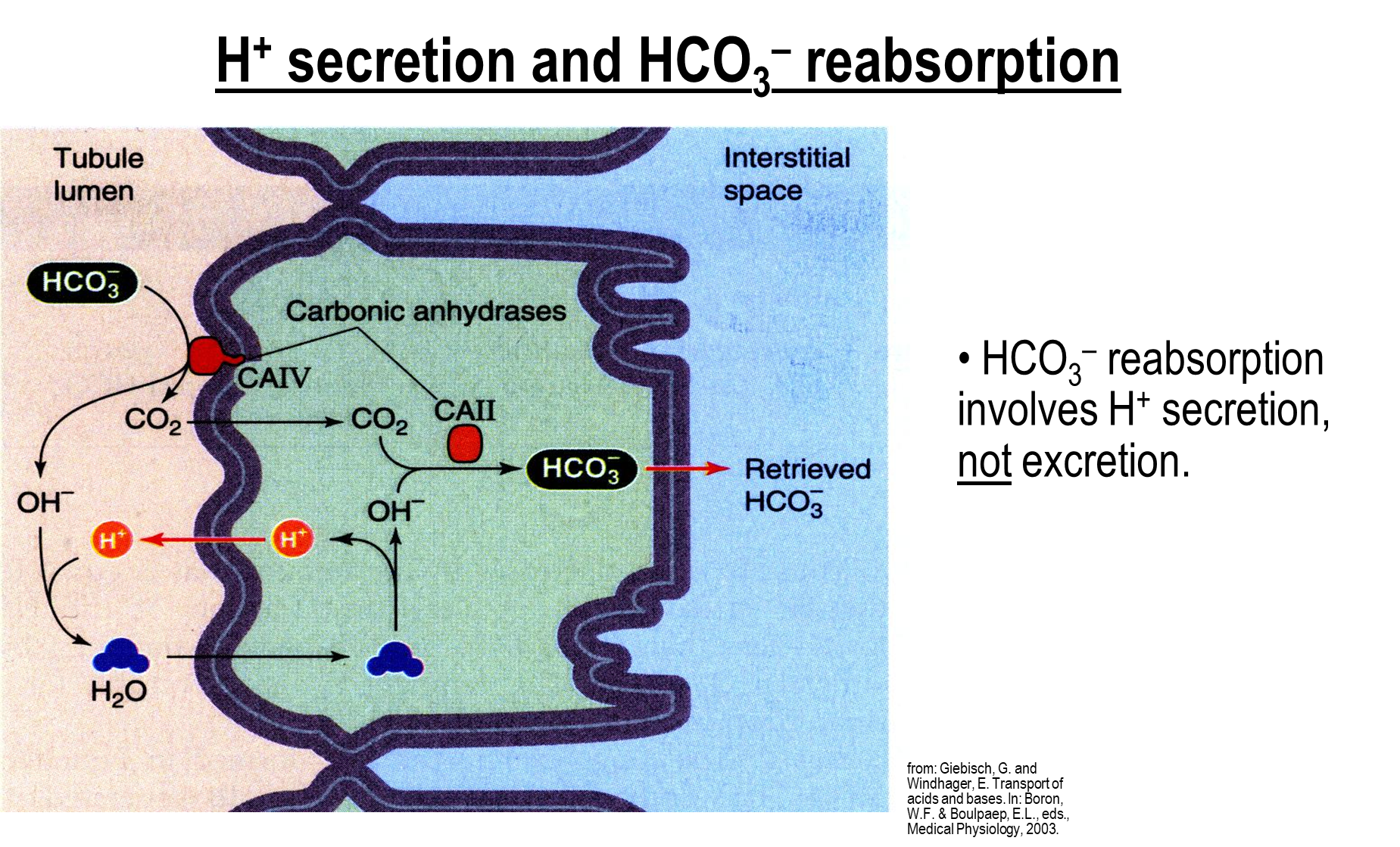
Bicarbonate reabsorption is dependent on the secretion of hydrogen ions rather than the excretion of hydrogen ions
1) At the proximal tubule, bicarbonate in the tubule lumen will be acted on by carbonic anhydrase IV on the apical membrane and be made into Hydroxide Ions and CO2
→ Hydroxide forms with a proton in the lumen into water
→ CO2 and water can freely cross into the cell
→ Water is then broken down into Hydroxide ions while the extra proton crosses out into the cell lumen
2) CO2 and Hydroxide Ions are reformed into bicarbonate by carbonic anhydrase II intracellularly which can then be crossed into the interstitial space
the secreted protons in this cycle will never end up being excreted in order to reabsorb bicarbonate
How does Hydrogen Excretion and Bicarbonate Regeneration occur with titratable acids?
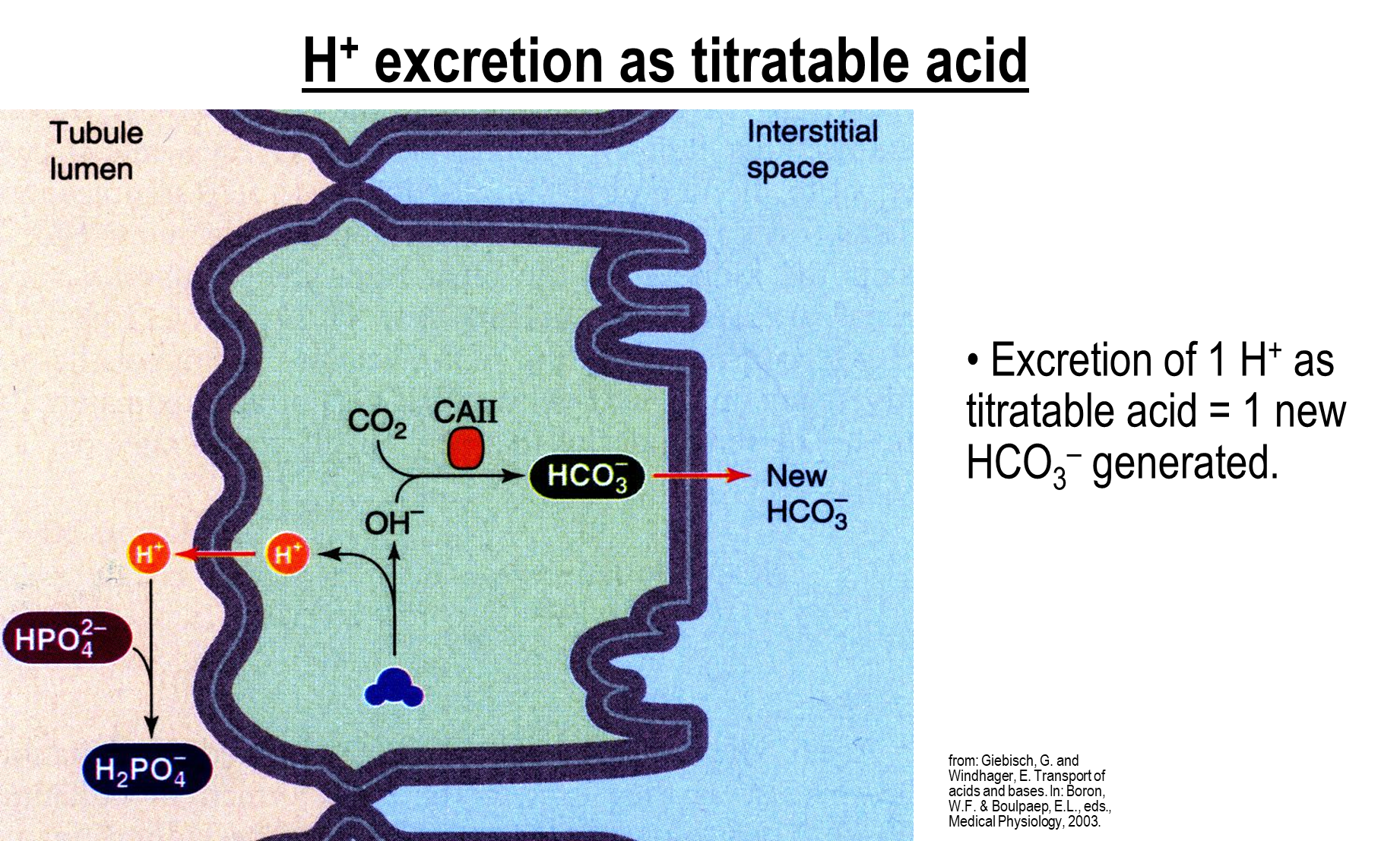
1) In the cell water is deprotonated, causing secretion of hydrogen into the tubule lumen
→ the hydrogen will bind to luminal buffers such as phosphate in order to form titratable acids
→ the protons will then be bound to the newly formed titratable acids and are secreted
2) In the cell the remaining hydroxide ion and CO2 are made into bicarbonate via the action of carbonic anhydrase II
→ this bicarbonate is then secreted into the interstitial space
How does Hydrogen Excretion work with Ammonium?
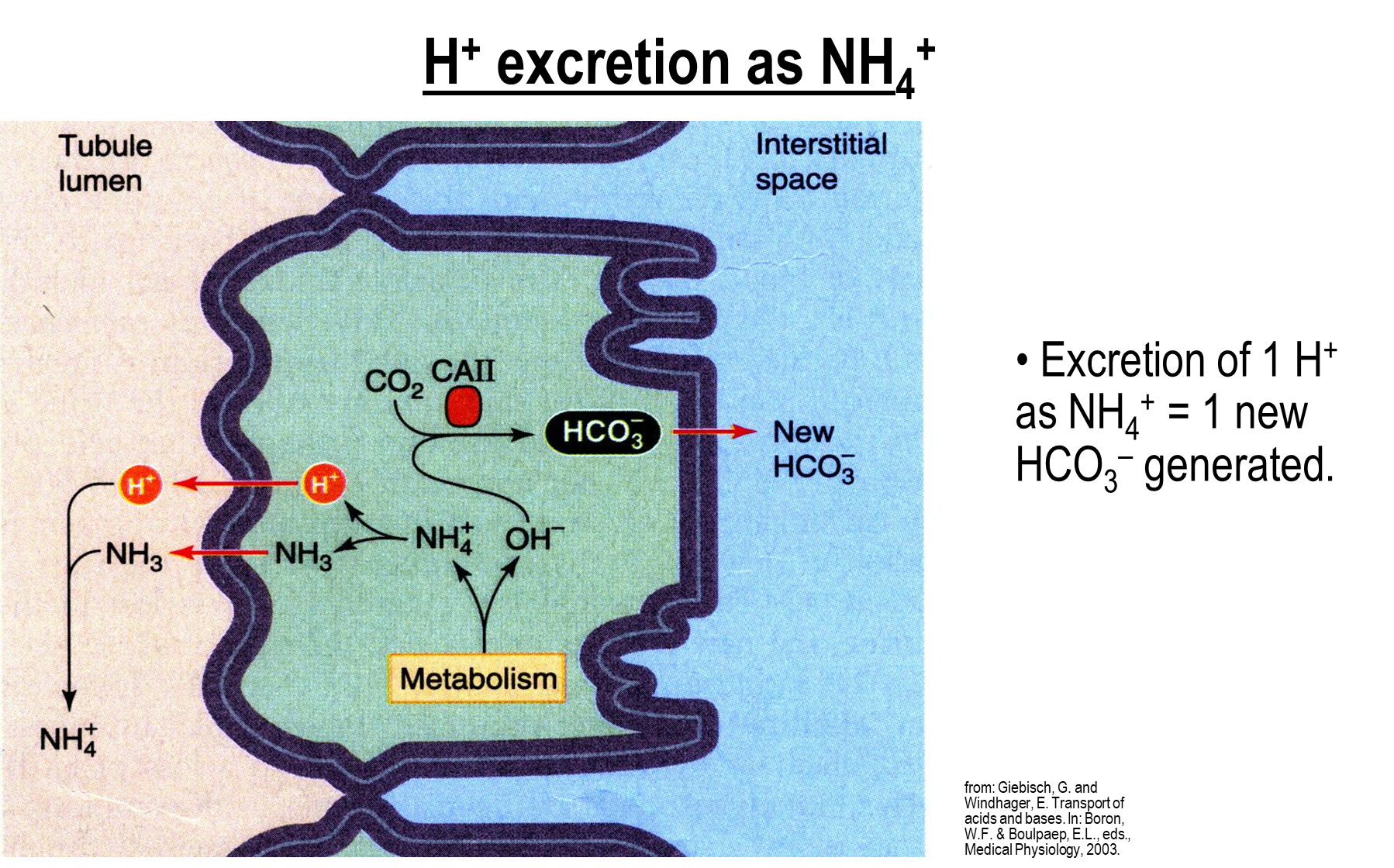
Occurs in the proximal tubule where metabolism of glutamine generates ammonium and hydroxide
1) Ammonium is broken down into ammonia and hydrogen ions which can then be secreted into the tubule lumen
→ the ammonium reforms in the tubule lumen and is excreted
2) Hydroxide will combine with CO2 with carbonic anhydrase II to make new bicarbonate which will be absorbed into the interstitial space
Where does bicarbonate reabsorption and generation take place along the nephron
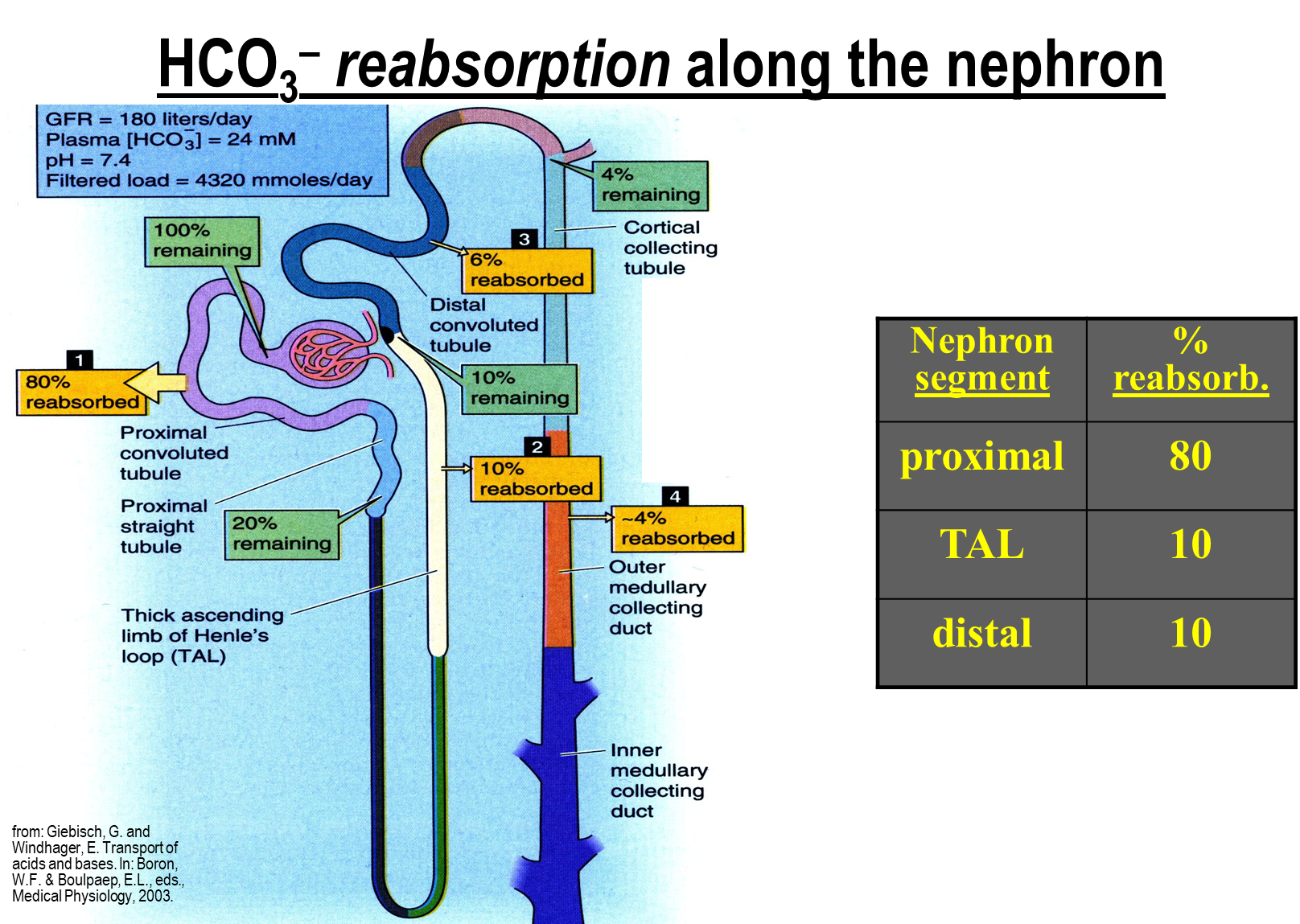
The majority of bicarbonate reabsorption takes place in the proximal tubule, with the remaining ten percent each taking place in the ascending limb and distal convoluted tubule
→ because of this acid excretion and new bicarbonate regeneration takes place in the proximal tubule
What is the main method of acid secretion in the proximal tubule?
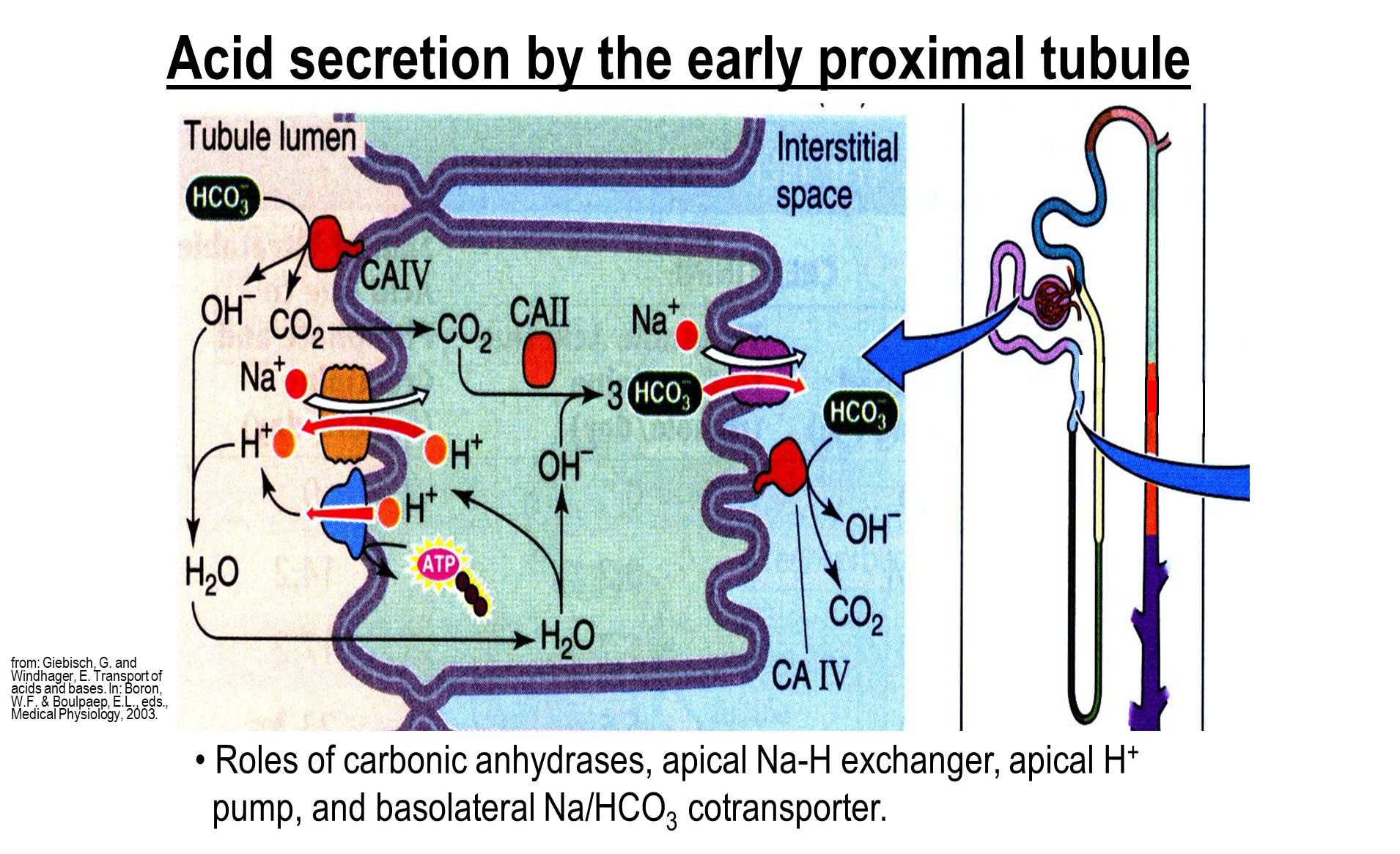
The major acid secretion method in the early and late proximal tubule is the sodium proton exchanger - these are the same across both early and late
1) The sodium proton exchanger aids in both sodium reabsorption and bicarbonate reabsorption by introducing large amounts of hydrogen ions into the lumen
2) Proton pump on the apical membrane
3) sodium bicarbonate cotransporter on the basolateral membrane
How does acid secretion occur in the thick ascending limb?
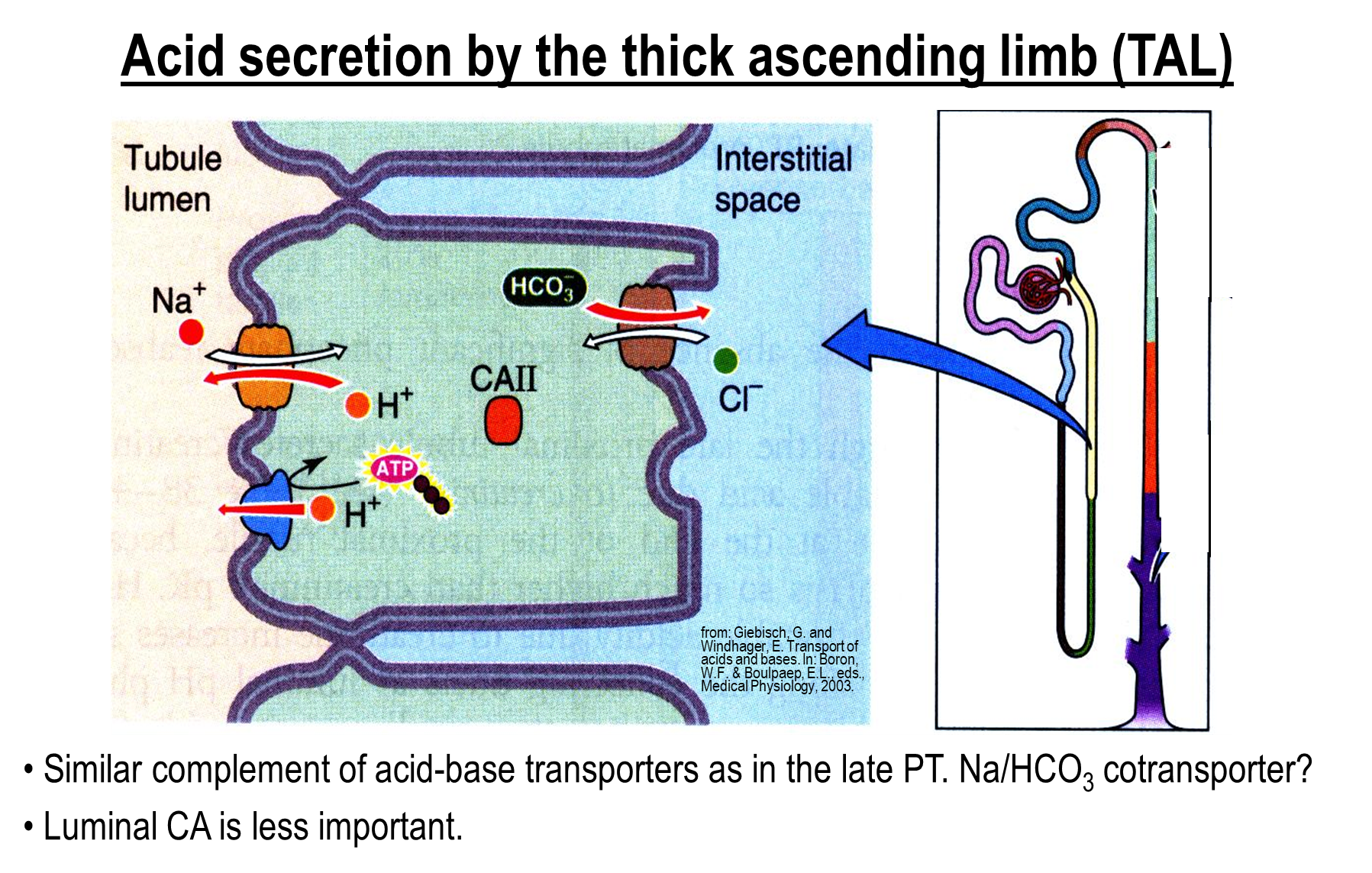
Around ten percent of bicarbonate is reabsorbed here. Follows the same mechanisms as the proximal tubule, except for the basolateral surface
1) Sodium Hydrogen Exchanger
2) Hydrogen Pump
3) Bicarbonate Chloride Exchanger on the basolateral surface
How does Acid-Base Secretion occur in the cortical collecting tubule and duct
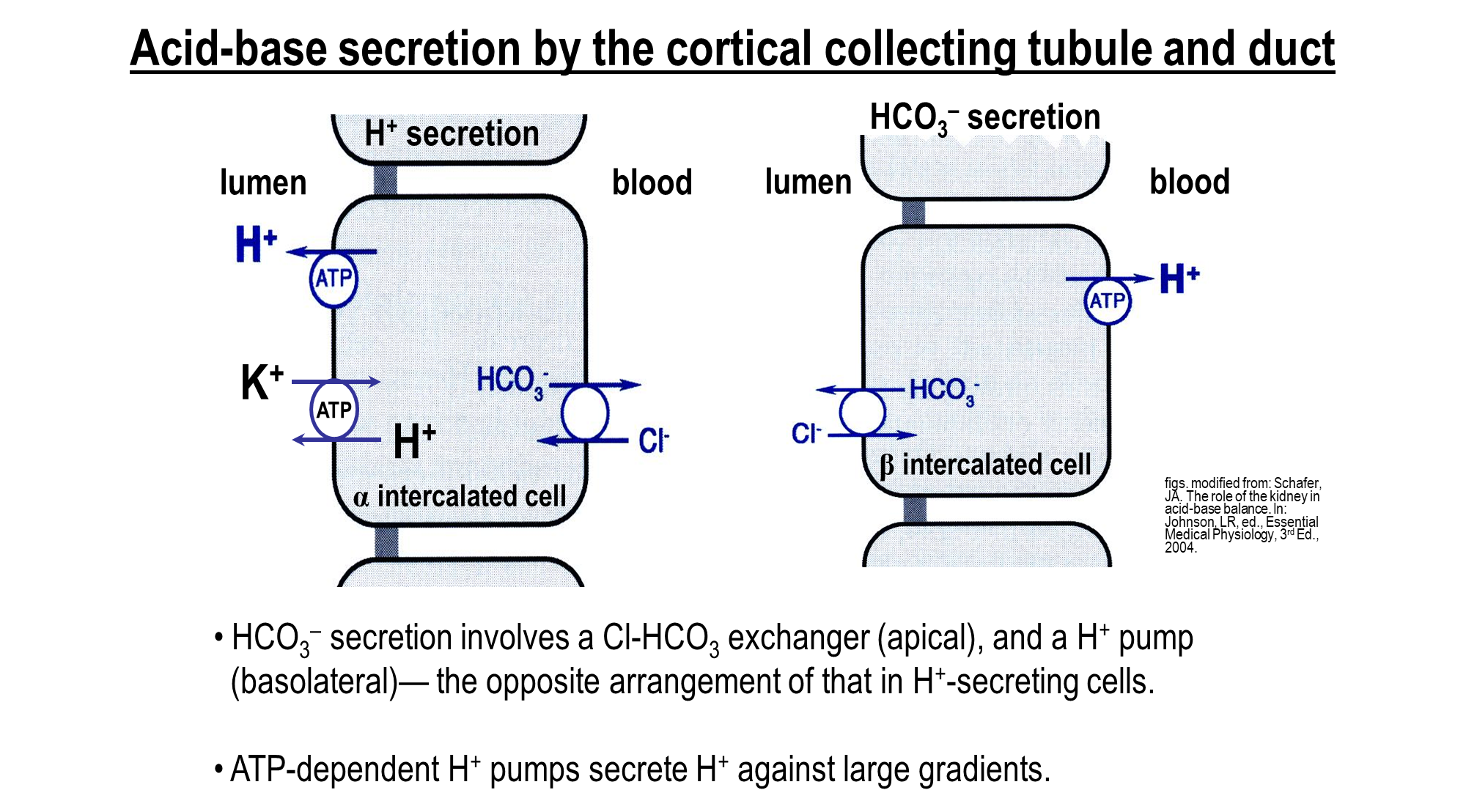
Alpha Intercalated Cells - uses ATP dependent mechanism in order to secrete hydrogen
→ Proton Pump
→ Hydrogen Potassium Exchanger
→ Bicarbonate and Chloride Exchanger (basolateral)
Beta Intercalated Cells - important in secretion of bicarbonate into the lumen
→ Bicarbonate Chloride Exchanger (apical)
→ Proton Pump (basolateral)
How does Ammonium Reabsorption occur in the thick ascending limb?
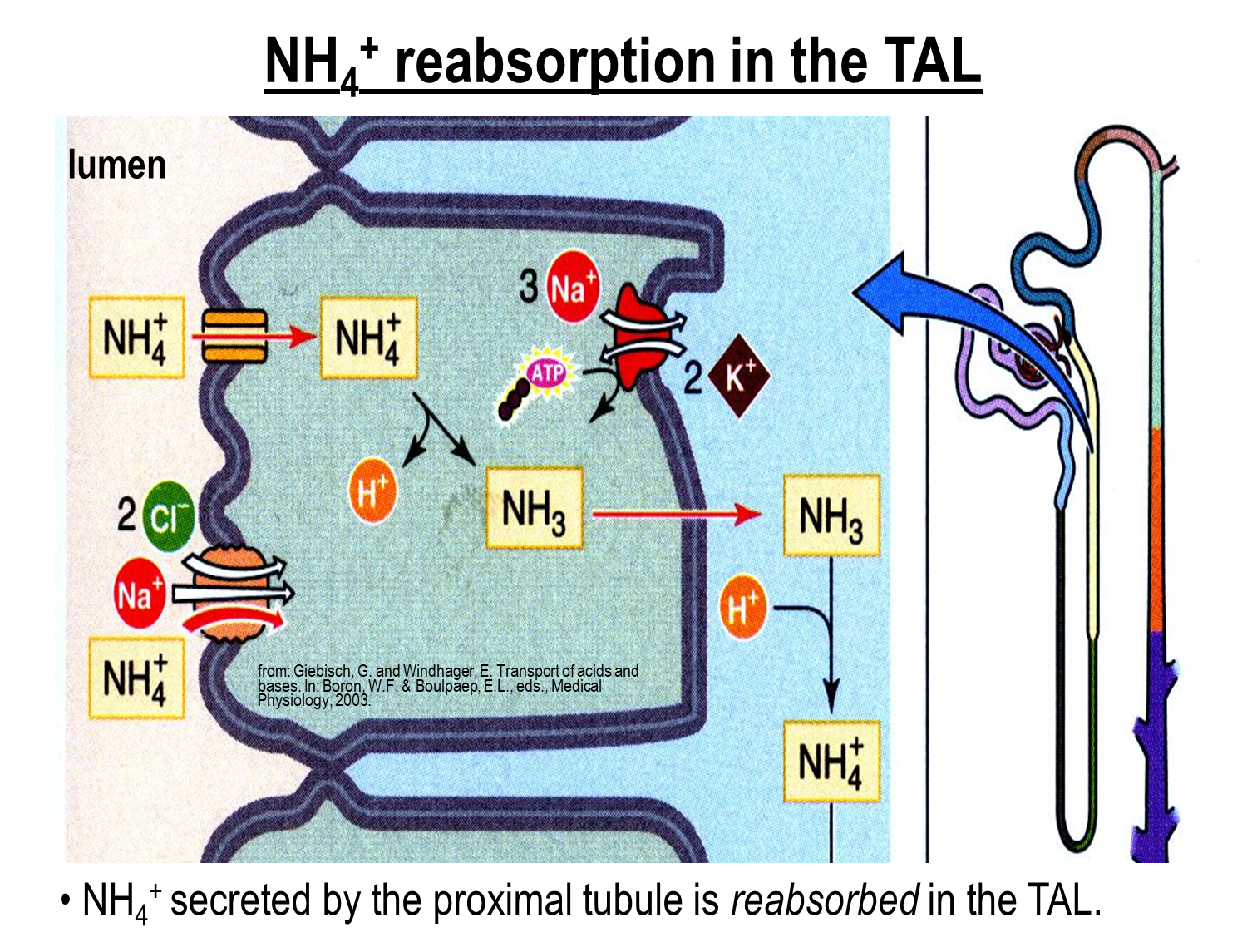
Ammonium generated in the proximal tubule is reabsorbed in the thick ascending limb
1) Ammonium is able to act as a substitute for potassium on both the apical potassium channel and the Na/K/2Cl channel on the surface of the ascending limb
→ this allows for ammonium to be reabsorbed and then deprotonate into ammonia
→ the ammonia will cross into the interstitium where it will reform as ammonium
Why does Ammonium recycling occur in the loop of Henle? (3)
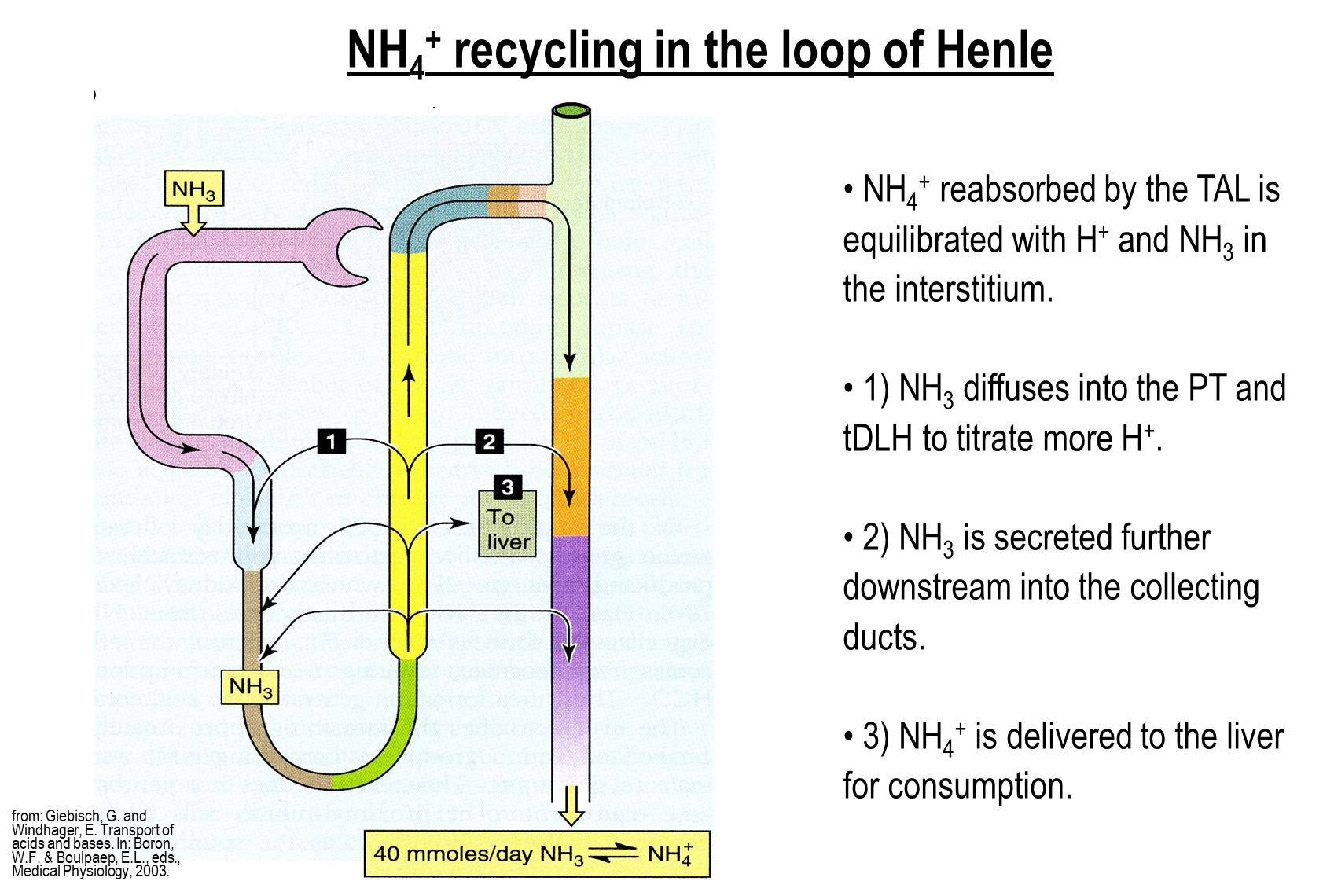
Ammonium generated in the proximal tubule is reabsorbed in the thick ascending limb. This is done for a variety of reasons
1) Ammonium reabsorbed is sent to the liver for urea generation
2) Ammonium may also be delivered to downstream nephron segments, allowing for us to bypass the cortical collecting tubules
→ this prevents reabsorption of the cortical collecting tubules, allowing for better excretion of ammonium
3) Some of the ammonium is delivered to upstream nephron segments such as the proximal tubule
What is the role of urea and bicarbonate in protein metabolism?
Protein processing produces nitrogenous waste, which can be excreted in the form of urea or ammonium which have opposite effects on acid base handling
1) In the liver, urea is formed from the reaction of ammonium and bicarbonate
→ loss of urea then means we lose bicarbonate
2) Ammonium loss in the proximal tubule will add a bicarbonate into the system as bicarbonate is needed for ammonium formation
→ conserves bicarbonate and balances the effect of urea
What are the four major acid base disorders?
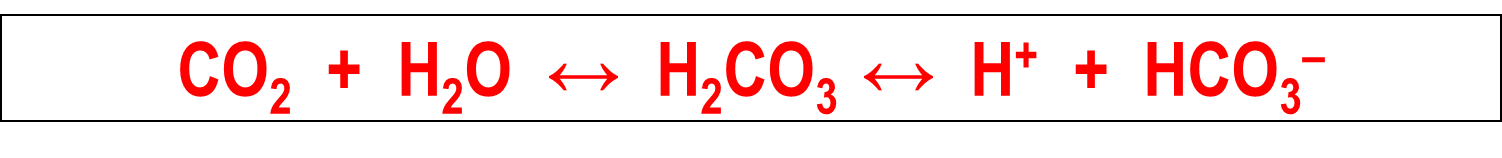
The four major acid-base disorders are based on an initial change to this formula driving the disorder
Respiratory Acidosis
→ increase in plasma CO2 results in a decreased pH and increased bicarbonate
Respiratory Alkalosis
→ decreased plasma CO2 results in increased pH and decreased bicarbonate levels
Metabolic Alkalosis
→ addition of alkali or massive loss of acid leads to a increase in PH and increase in bicarbonate
Metabolic Acidosis
→ addition of acids leads to an decrease in pH and a decrease in bicarbonate
How does compensation in acid base disorders occur?
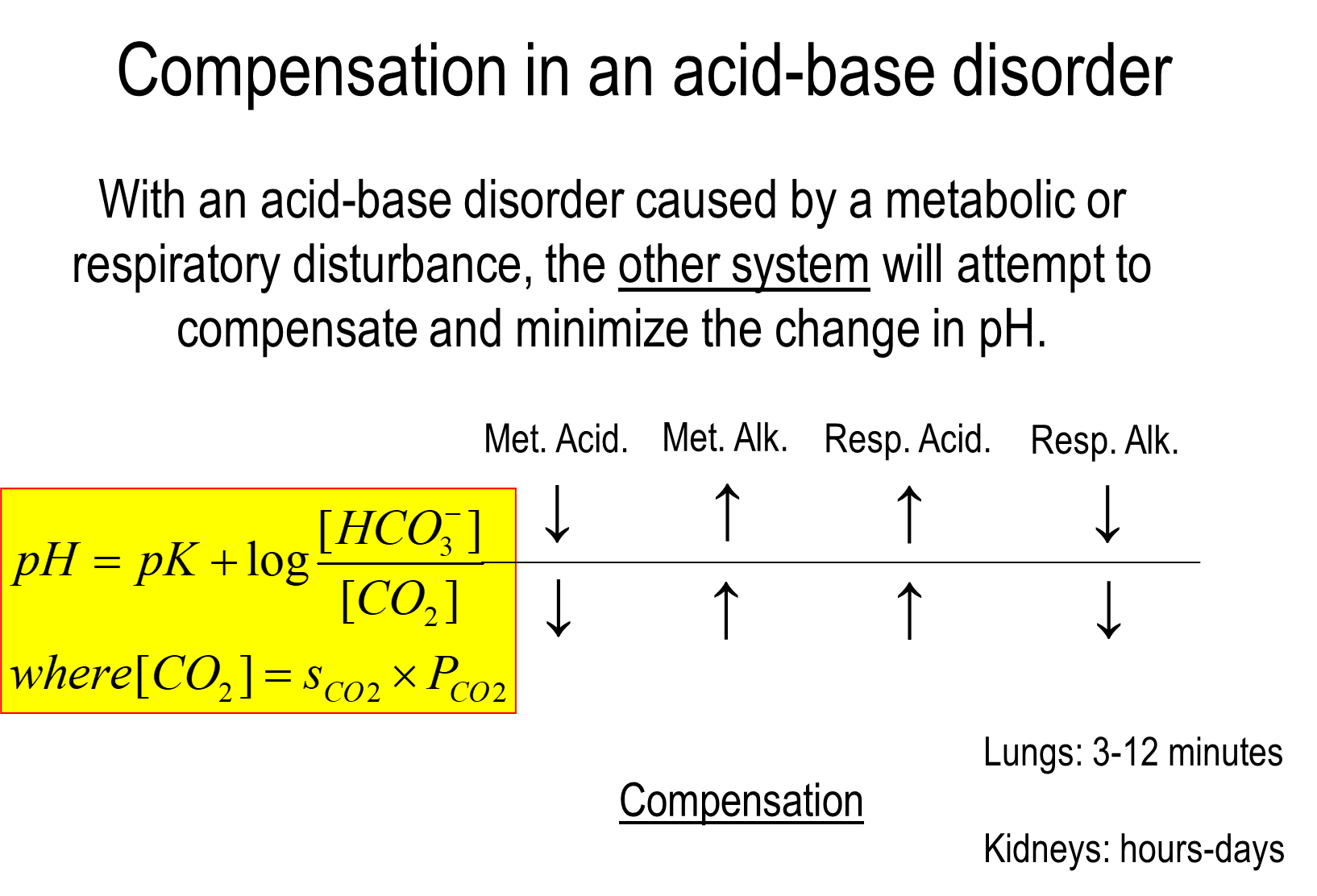
During an acid base disorder caused by either the lung or the kidney, the other system will attempt to compensate and minimize pH changes
1) Metabolic Acidosis
→ drop in bicarbonate levels leads to a fall in CO2 as well in order to correct for the decreased pH
2) Metabolic Alkalosis
→ increase in bicarbonate levels leads to an increase in CO2 in order to correct for increased pH
3) Respiratory Acidosis
→ increase in CO2 levels will result in a compensation of the kidney by increasing bicarbonate production
4) Respiratory Alkalosis
→ decrease in CO2 levels will result in renal compensation by decreasing bicarbonate production
lungs compensate quickly while kidneys take hours to days
What are the effects of acidosis on renal acid secretion?
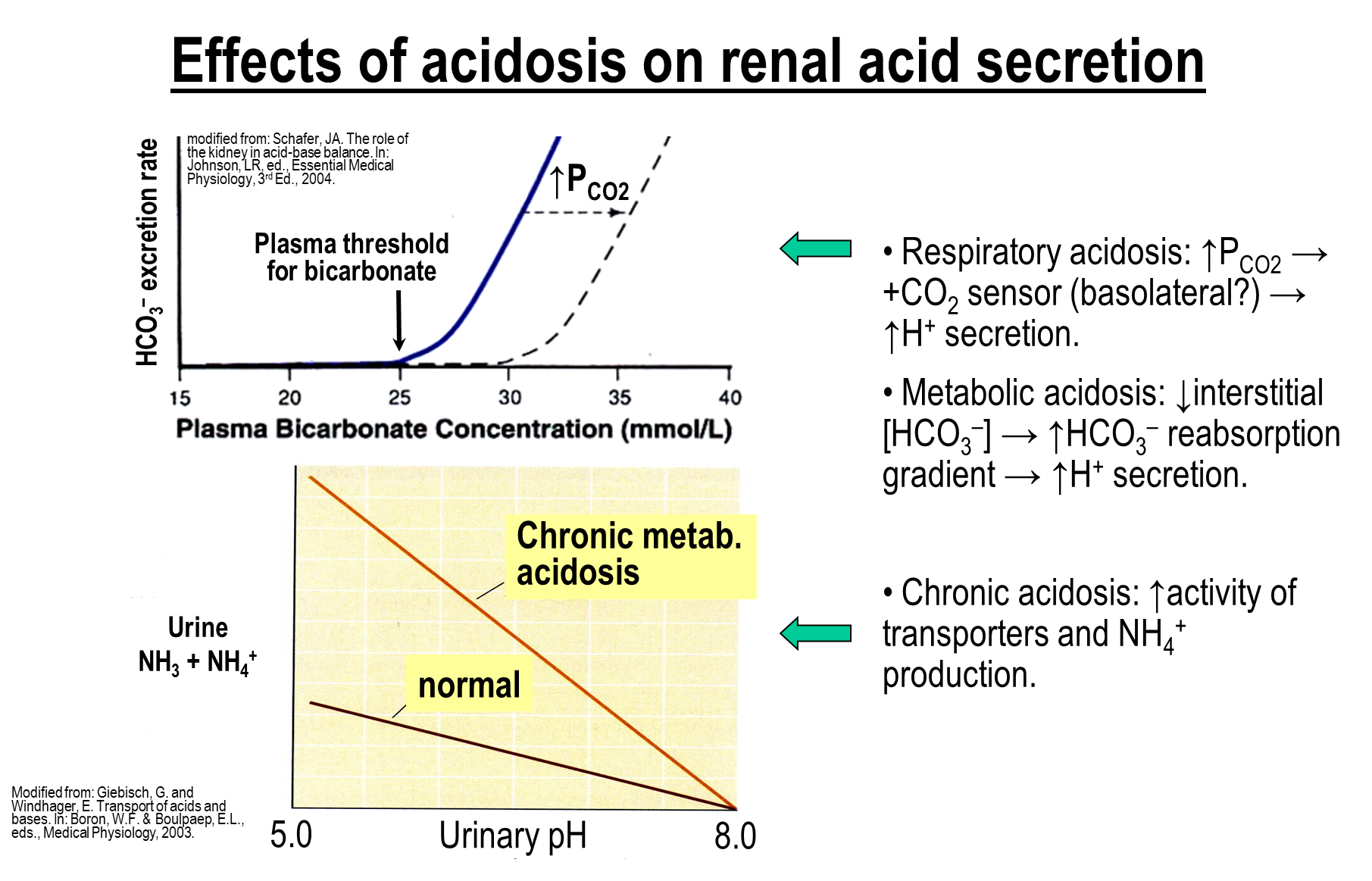
Respiratory Acidosis
→ increase in PCO2 will shift the bicarbonate excretion rate to the right
→ at any given plasma bicarbonate concentration there is less bicarbonate being excreted - more bicarbonate being reabsorbed
Metabolic Acidosis
→ drop in bicarbonate concertation leads to an increase in sodium and bicarbonate reabsorption
Chronic Acidosis
→ increased ammonium production
What are the effects of alkalosis on renal acid secretion
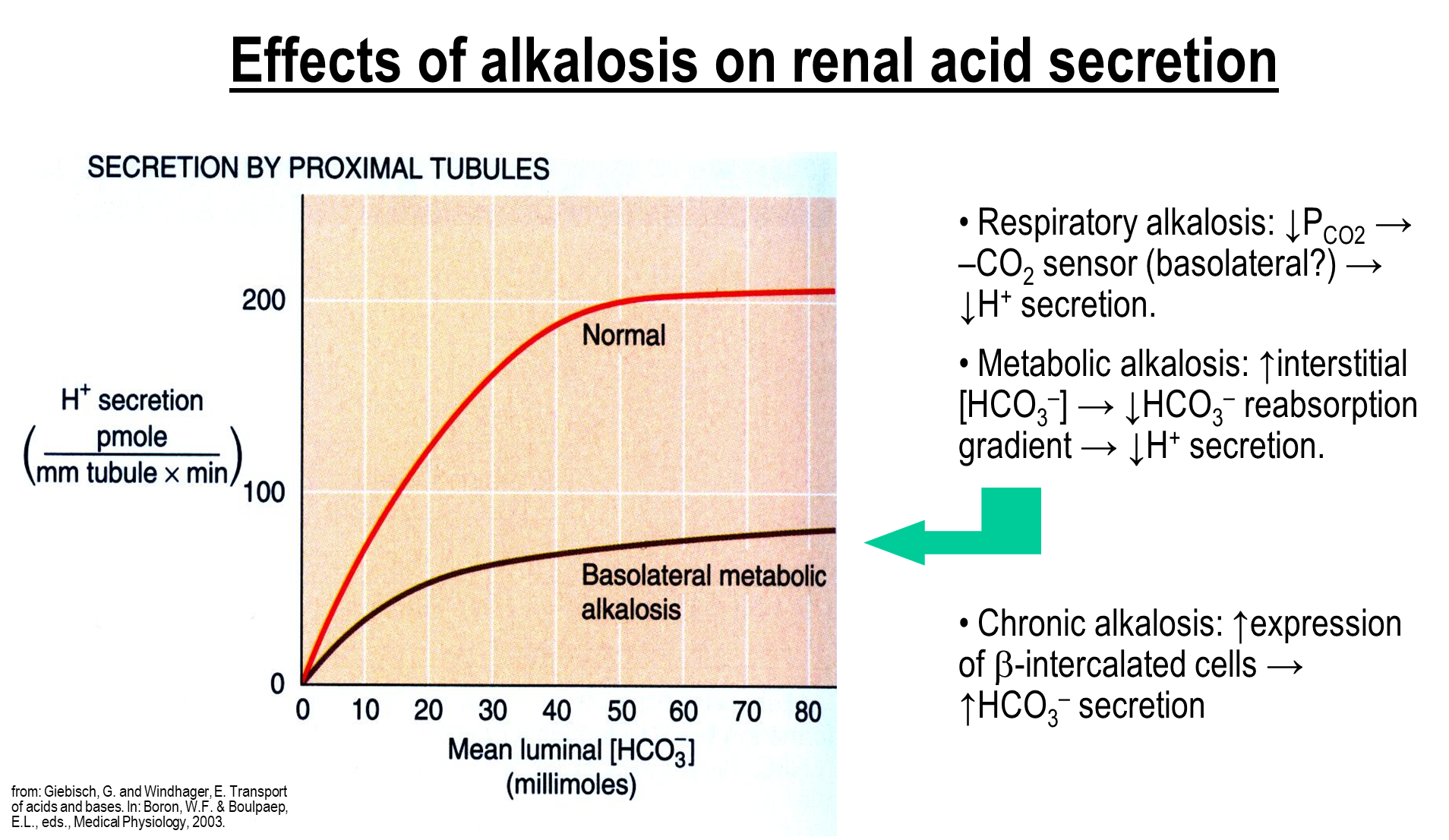
Respiratory Alkalosis
→ drop in PCO2 leads to increased bicarbonate excretion
Metabolic Alkalosis
→ increase in bicarbonate leads to decreased bicarbonate reabsorption and hydrogen secretion
Chronic Alkalosis
→ increased expression of beta-intercalated cells leading to increased bicarbonate secretion
What is the Davenport Diagram
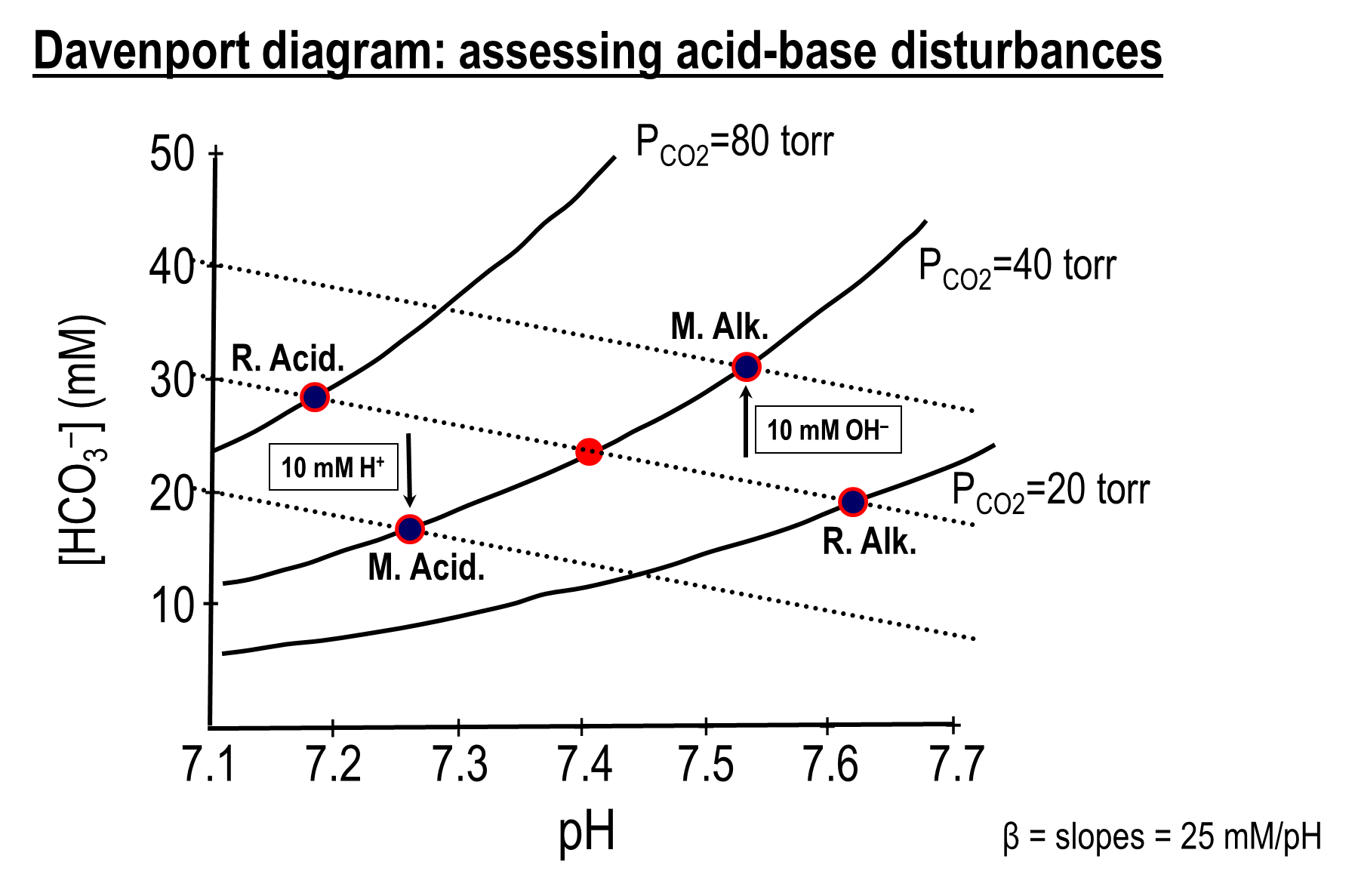
graphical tool used in physiology to represent the relationship between blood pH, bicarbonate concentration, and partial pressure of CO2, allowing us to visualize acid-base disturbances
1) Based on the Henderson Hasselbalch equation
→ pH = pK + log [HCO3]/[CO2]
2) Based on the diagram, in the absence of any buffering systems an increase in PCO2 at the same bicarbonate level would drop the pH significantly
→ in the same line, with infinite number of non-bicarbonate buffering systems would maintain the same pH with an increase in CO2, but the bicarbonate level would increase
→ the slope in between these two imaginary lines would present the effect of non-bicarbonate buffering