Medicinal Chemistry of Antiviral Drugs
1/90
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
91 Terms
What are good antiviral drug targets?
efficient - important to the life cycle of the virus
selective and safe - bear little resemblance to human proteins
broad spectrum - common to a variety of different viruses
important to the early stages of the viral life cycle
Which viruses are DNA viruses?
herpes simplex virus
type 1 infection - mouth, face, skin, brain
type 2 infection - genitals, rectum, skin, hands, meninges
varicella-zoster virus
chickenpox in children
shingles in adults
cytomegalovirus
life-threatening for immunocompromised patients
What are the types of drugs used to treat DNA viruses?
viral DNA polymerase inhibitor
tubulin polymerization inhibitor
What is the role of nucleoside triphosphate in DNA replication?
they serve as building blocks for forming a new DNA strand using a DNA template which is catalysed by DNA polymerase
What enzyme catalyses the formation of new DNA strands during replication?
DNA polymerase
Why are nucleoside analogues used in antiviral therapy?
effective against DNA virus
especially herpes viruses
How do nucleoside analogues inhibit DNA polymerase?
mimics natural nucleosides
they get incorporated into viral DNA and terminate chain elongation
Why are herpes viruses particularly susceptible to nucleoside analogues?
they rely heavily on viral DNA polymerase for replication which the nucleoside analogues target
What type of drug is acyclovir?
a nucleoside analogue
Which natural nucleoside does aciclovir resemble and what structure does aciclovir lack compared to the natural nucleoside?
resembles deoxyguanosine
it lacks the complete sugar ring
How is aciclovir activated in the body?
phosphorylated in three stages to form the active triphosphate form
What is the mechanism of action of acyclovir triphosphate?
inhibits viral DNA polymerase and causes chain termination during DNA replication
How to nucleoside analgouses cause chain termination?
they incorporate into growing DNA strand but lack a 3’-OH group which prevents further elongation
Why is aciclovir selectively taken up by infected cells?
infected cells have mechanisms that absorb aciclovir over healthy cells
What enzyme activates acyclovir in infected cells?
viral thymidine kinase
100 x more effective than host thymidine kinase
phosphorylates aciclovir to its monophosphate form, initiates activation
What are the uses for acyclovir?
herpes simplex 1 and 2 infections
varicella zoster virus
Is acyclovir active against all herpes types?
no due to mutation in thymidine kinase or DNA polymerase enzymes
also not effective in Cytomegaloviruses it has no viral thymidine kinase
which is needed to activate acyclovir
Why does acyclovir have low oral bioavailability?
due to poor intestinal permeability
only 15-30% is absorbed
therefore prodrugs are used to increase interstinal permeability
What is Valaciclovir and how does it enhance bioavailability?
an l-valyl ester prodrug of acyclovir which is designed to improve absorption
uses active transport via HP1-1 protein which increases bioavailability to 54%
Where is Valaciclovir converted to Aciclovir?
in liver and gut wall through hydrolysis
What is the clinical use of valacyclovir?
varicella-zoster virus
What is ganciclovir used to treated?
Cytomegalovirus (CMV) infections.
How is Ganciclovir activated in CMV-infected cells?
By kinases other than thymidine kinase, converting it to its monophosphate form.
Why does Ganciclovir have low oral bioavailability?
It is highly polar, leading to poor intestinal permeability.
What is Valganciclovir and how does it improve bioavailability?
It is a valyl prodrug of Ganciclovir that enhances absorption via active transport mechanisms.
What type of drug is podophyllotoxin and where is it derived from?
Tubulin polymerization Inhibitor
plant-derived product
What is Podophyllotoxin used to treat?
Genital warts caused by the DNA virus papillomavirus.
What are the two main actions of Podophyllotoxin?
Antiviral and antimitotic.
How does Podophyllotoxin exert its antimitotic effect?
By inhibiting tubulin polymerization, disrupting microtubule formation during cell division.
What are the challenges in treating HIV?
no vaccination available
as virus keeps mutating
antiviral drugs slow disease but don’t eradicate disease
virus undergoes many mutations easily
this results in rapid resistance to antiviral drugs
What are the key enzymes involved in the HIV life cycle and what are their roles?
reverse transcriptase
catalyses synthesis of DNA strand using viral RNA as template = RNA/DNA template
then it catalyses the degradation of RNA strand
uses remaining DNA strand as template to catalyse the synthesis of dsDNA
integrase
inserts viral DNA into host cell’s DNA
protease
cleaves polyproteins into functional viral proteins for assembly
What type of antiviral drugs are used to treat HIV?
reverse transcriptase inhibitors
protease inhibitors
integrase inhibitors
fusion inhibitors
Why is reverse transcriptase a key drug target in HIV treatment, and what challenge does it present?
unique to HIV - ideal for selective inhibition
Challenge:
its still a DNA polymerase so there is risk of cross inhibition with host cellular DNA polymerases therefore drug design must balance potency against HIV with minimal toxicity to host cells
What are the types of reverse transcriptase inhibitors?
nucleoside reverse transcriptase inhibitors (NRTIs)
non-nucleoside reverse transcriptase inhibitors (NNRTIs)
How do NRTIs become active and why is this activation necessary?
they are phosphorylated by 3 cellular enzymes which forms an active nucleoside triphosphate a
this is necessary as HIV lacks a viral kinase so it relies on host enzyme for activation
What structural modification makes Zidovudine a chain terminator?
The 3'-OH group in deoxythymidine is replaced by an azido group (N₃), preventing DNA chain elongation
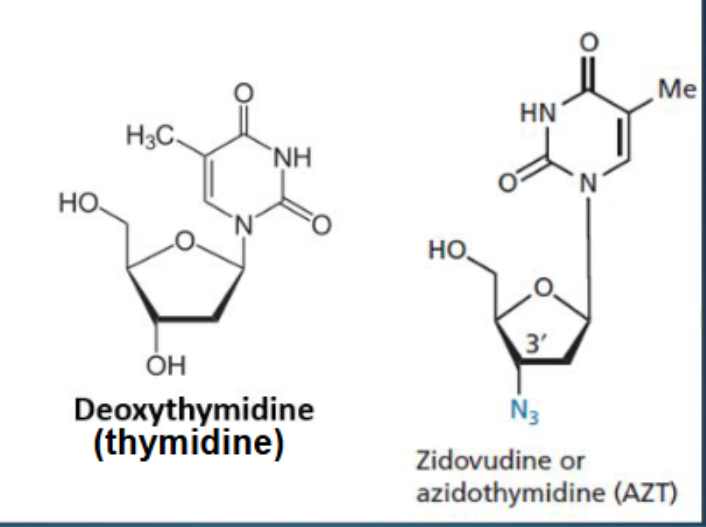
How does Zidovudine inhibit HIV replication?
once phosphorylated to its triphosphate form it inhibits reverse transcriptase and terminates DNA synthesis by attaching to growing DNA chain by terminating the chain
Why is Zidovudine considered selective for HIV?
It has greater affinity for viral reverse transcriptase than for human DNA polymerase.
What are potential side effects of Zidovudine?
anemia
What structural change is common to Lamivudine and Emtricitabine?
both replace the 3' carbon in the sugar ring with sulfur, preventing DNA chain elongation.
What distinguishes Lamivudine from Emtricitabine chemically?
Lamivudine has R = H
Emtricitabine has R = F.
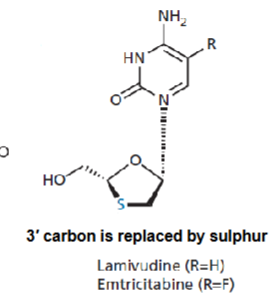
How do deoxycytidine analogues inhibit HIV replication?
After phosphorylation to triphosphate form, they inhibit reverse transcriptase and act as chain terminators.
Which deoxycytidine analogue is also approved for hepatitis B treatment?
lamivudine
Why do deoxycytidine analogues act as chain terminators?
they lack a 3'-OH group in the sugar ring, so DNA synthesis cannot continue.
Why are NNRTIs considered less toxic than NRTIs?
NNRTIs have higher selectivity for HIV-1 reverse transcriptase over host DNA polymerases → fewer side effects
What is a major limitation of NNRTIs in HIV treatment?
Rapid resistance can develop due to mutations in the NNRTI binding site.
Why are NNRTIs and NRTIs often combined in HIV therapy?
Combining from the start reduces resistance risk; they bind to distinct sites on reverse transcriptase.
Do NNRTIs require phosphorylation for activation like NRTIs?
NNRTIs bind directly to reverse transcriptase without needing phosphorylation.
What are the first generation NNRTIs?
nevirapine
delavirdine
efavirenz
What are the second generation NNRTIs?
etravirine
rilpivirine
What structural feature and binding interactions define Nevirapine as a first generation NNRTI?
rigid butterfly like conformation
interacts with HIV-1 reverse transcriptase at key residues
What is nevirapine a derivative?
Dipyridino-diazepine derivative
How does Nevirapine’s left wing interact with HIV-1 reverse transcriptase?
it binds to aliphatic residues: Leu-100, Val-106, Val-179.
What interactions occur at Nevirapine’s right wing?
Hydrophobic and van der Waals interactions with aromatic residues: Tyr-181, Tyr-188.
Why is Nevirapine vulnerable to resistance?
Mutations at its binding residues (e.g., Tyr-181, Tyr-188) can disrupt its interaction with reverse transcriptase.
What does the rigid butterfly-like conformation of Nevirapine imply for drug design?
Enhances binding specificity (potency) but limits flexibility, making it more susceptible to resistance mutations.
What is metabolic autoinduction in the context of Nevirapine?
Nevirapine induces its own metabolism, reducing its half-life from 45 to 23 hours after 2–4 weeks.
Why is Nevirapine combined with NRTIs in HIV therapy?
to prevent resistance development, as NNRTIs alone are prone to rapid resistance.
What was the primary goal in developing second-generation NNRTIs?
To target both wild-type HIV and variants resistant to first-generation NNRTIs.
Name two second-generation NNRTIs and their brand names and what chemical class do they belong to?
Etravirine (Intelence)
Rilpivirine (Edurant)
chemical class - Diarylpyrimidine (DAPY) derivatives.
How do second-generation NNRTIs improve resistance handling?
their flexible structures allow better accommodation of mutations in the reverse transcriptase binding site.
What enzyme family does HIV-Protease belong to?
Aspartyl proteases.
Describe the structural composition of HIV-Protease?
symmetrical dimer of two identical subunits (99 amino acids each), resembling an English bulldog face.
What makes the HIV-Protease active site unique compared to human proteases?
it is symmetrical → allows for selective drug targeting.
Which amino acids form the catalytic region of HIV-Protease?
Asp25, Thr26, Gly27 — located at the floor of the active site.
What is the role of the flap region in HIV-Protease?
Each monomer provides a flap that closes over the substrate once bound.
How many binding subsites does HIV-Protease have and which subsites are crucial for inhibitor interaction?
Eight total subsites: S4–S1 and S1'–S4' (four per monomer).
crucial - S2–S2' is key for binding and inhibition.
What type of peptide bond does HIV-Protease cleave?
Aromatic–proline bond, typically between phenylalanine or tyrosine and proline
Why is HIV-Protease selective compared to mammalian proteases?
Mammalian proteases do not cleave aromatic–proline bonds → allows selective inhibition.
Why are aspartic acids crucial in the catalytic mechanism of HIV protease?
bridge a water molecule that performs nucleophilic attack during amide bond hydrolysis.
What intermediate is formed during HIV-Protease catalysis?
A tetrahedral intermediate — representing the transition state of the reaction
What are the final products of HIV-Protease cleavage?
A carboxylic acid and an amine — resulting from peptide bond hydrolysis.
Why is the catalytic mechanism of HIV-Protease a key drug target?
inhibitors mimic the tetrahedral intermediate, blocking the enzyme’s function and halting viral maturation.
What is the design strategy behind HIV protease inhibitors?
they are designed as transition-state inhibitors to mimic the enzyme’s catalytic transition state.
Give an example of a HIV Protease inhibitor?
Saquinavir
What stereochemistry is essential for Saquinavir’s activity?
R-stereochemistry is crucial; S-configuration results in complete loss of activity.
What was the key design strategy used to improve Saquinavir’s potency?
stepwise optimization of binding groups and incorporation of a transition-state isostere to mimic the tetrahedral intermediate.
What are the disadvantages of Saquinavir?
high molecular weight and peptide character
poor absorption, metabolic susceptibility, rapid excretion, limited access to CNS, high plasma protein binding = poor bioavailability
susceptible to resistance development
What is a limitation of using HIV protease inhibitors (PIs) alone?
like reverse transcriptase inhibitors, protease inhibitors offer short term benefit alone due to rapid resistance development
How does combination therapy improve HIV treatment outcomes?
combing PIs and RTIs increases antiviral activity and slows resistance development
What is the role of HIV integrase inhibitors in viral replication?
They block the integration of viral DNA into the host genome, halting HIV replication.
Name three HIV integrase inhibitors and their brand names.
Raltegravir (Isentress)
Elvitegravir (Stribild)
Dolutegravir (Tivicay)
Why are integrase inhibitors considered highly effective in HIV therapy?
They target a unique viral enzyme with minimal cross-reactivity to human enzymes → high selectivity and potency.
What is the mechanism of action of Raltegravir?
It chelates a divalent cation (Mn²⁺) at the active site of HIV integrase, blocking DNA integration.
Which structural feature of Raltegravir is crucial for metal chelation?
Coplanarity between the ketone and β-carbonyl enhances chelation of Mn²⁺.
What is the role of fusion inhibitors in HIV therapy?
they prevent HIV-1 from binding and fusing with host cells, blocking viral entry.
What is Maraviroc’s mechanism of action?
It acts as a CCR5 antagonist, blocking the coreceptor on host cells and preventing viral fusion.
What makes Maraviroc unique among anti-HIV drugs?
It targets a host cell protein (CCR5), not the virus itself — first of its kind.