Electron/Molecular Geometry and Hybridizations
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
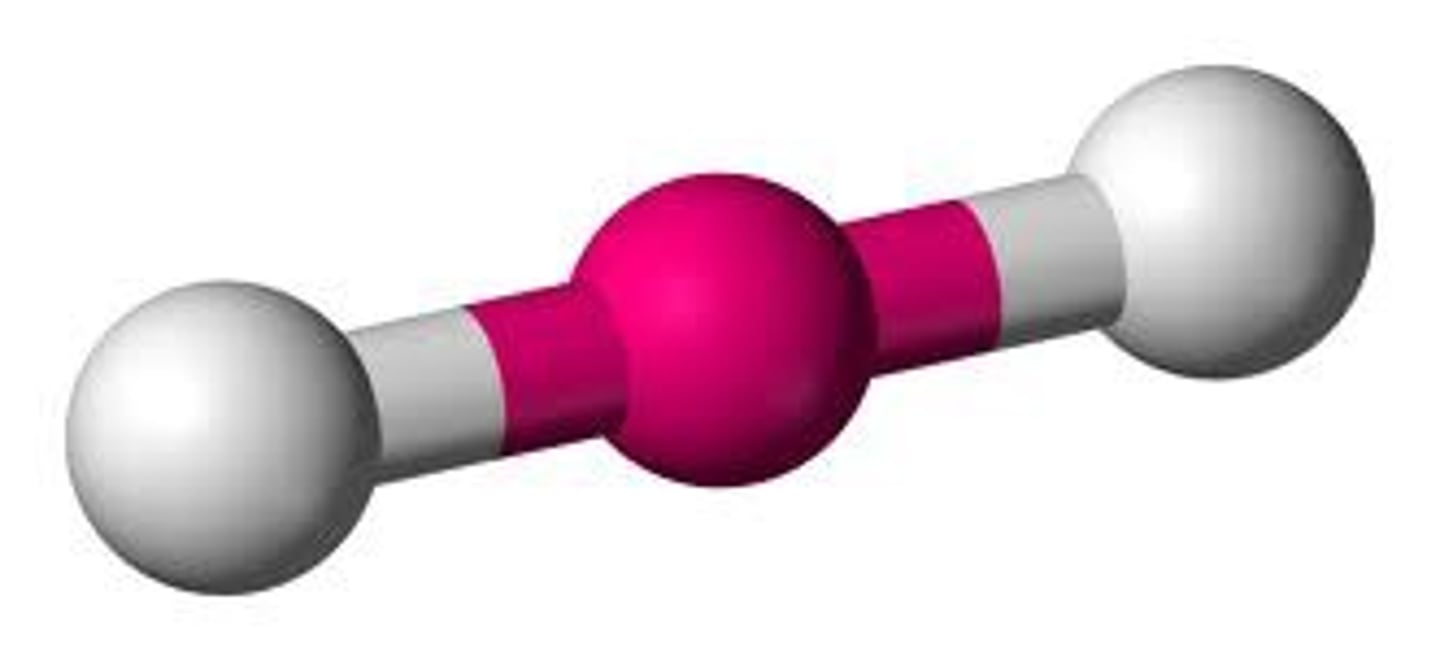
Linear
Electron group arrangement of 2 electron groups (AX2). Forms an ideal bond angle of 180 degrees (Ex. CO2, BeCl2)
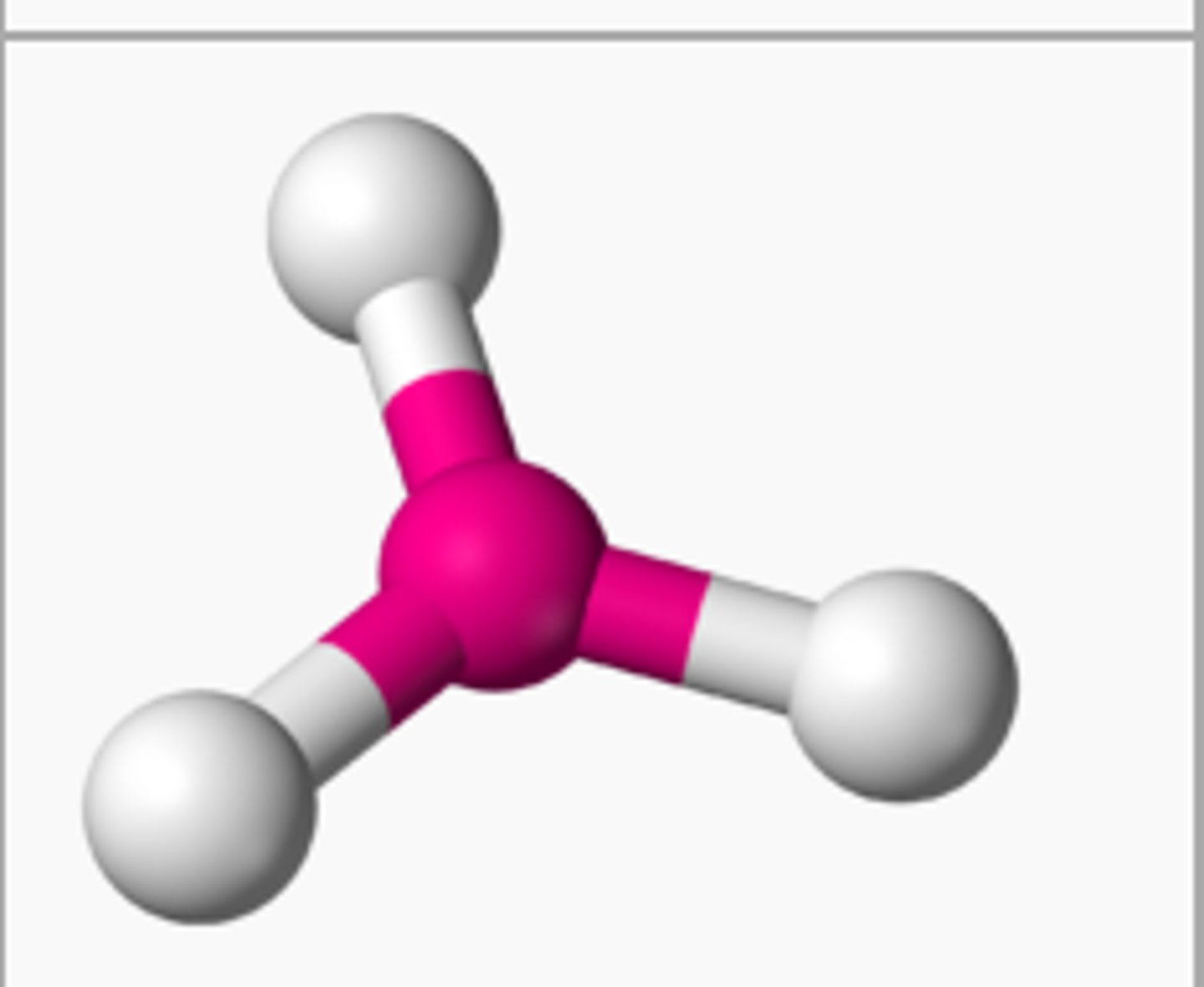
Trigonal Planar
Electron group arrangement of 3 electron groups (AX3). Forms ideal bond angles of 120 degrees (Ex. NO3-, SO3)
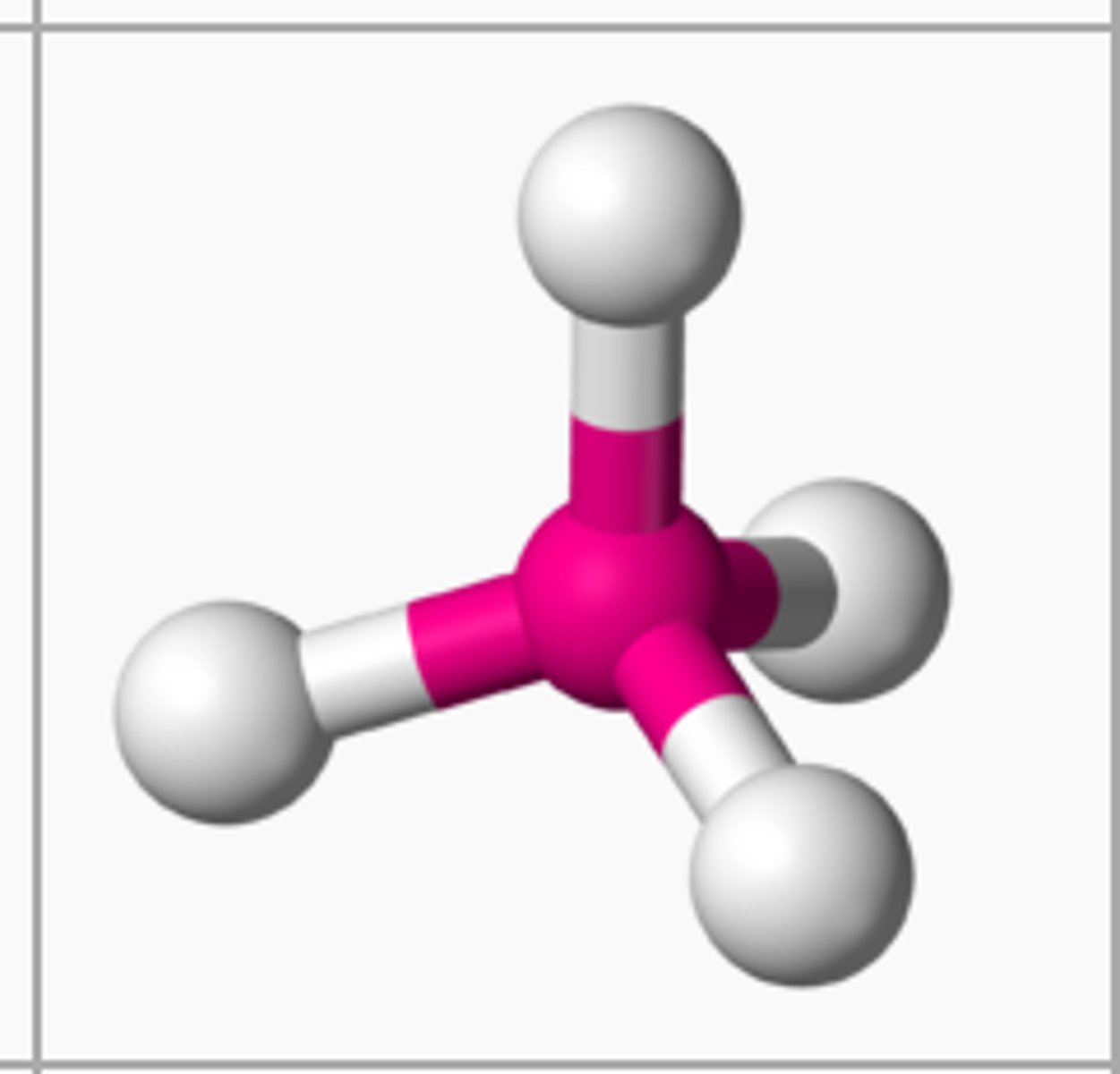
Tetrahedral
Electron group arrangement of 4 electron groups (AX4). Forms ideal bond angles of 109.5 degrees (Ex. CH4, SiCl4, ClO4-)
Trigonal Bipyramidal
Electron group arrangement of 5 electron groups (AX5). Forms ideal bond angles of 120 degrees (equatorial) and 90 degrees (axial) (Ex. PCl5, SOF4)
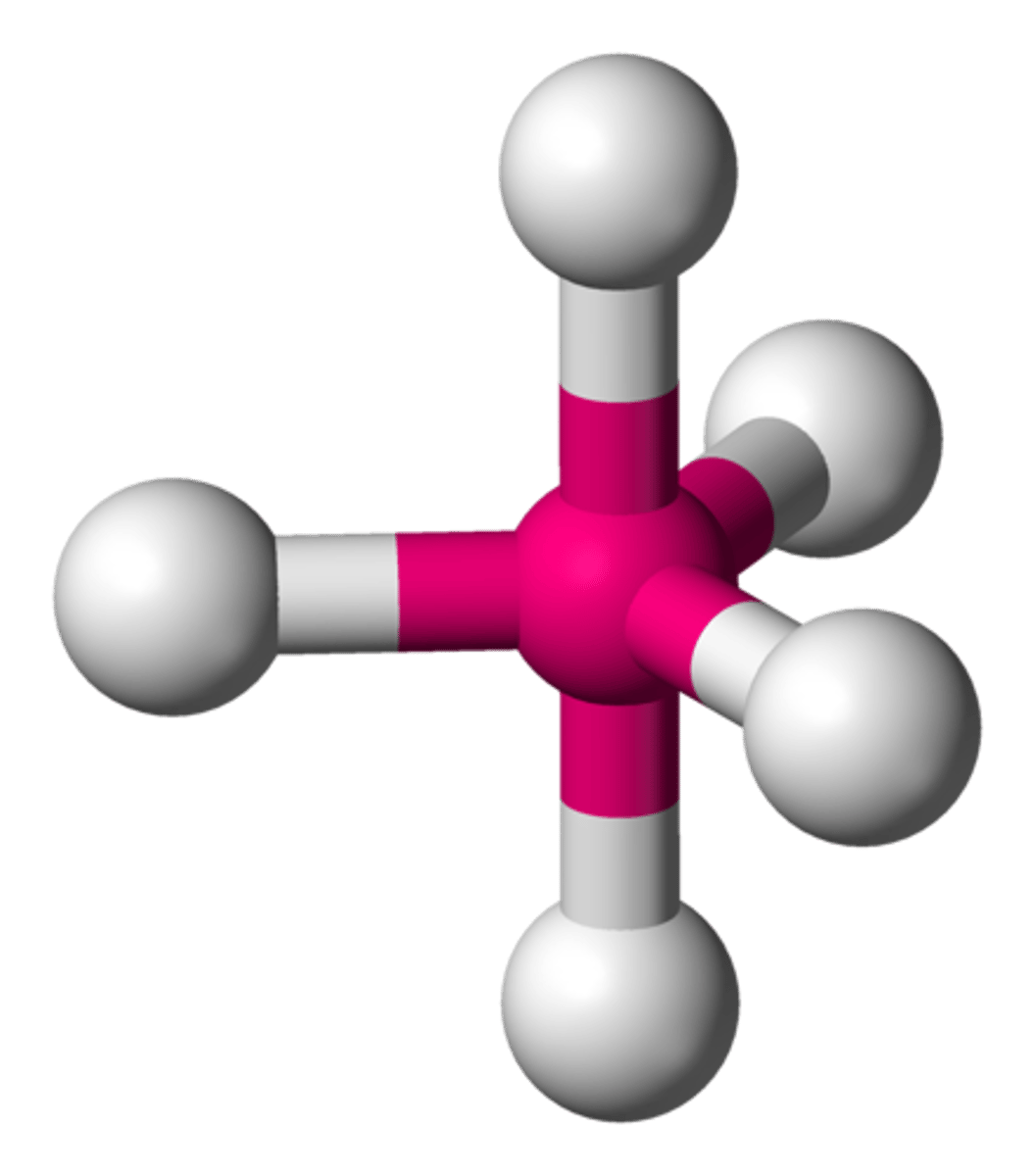
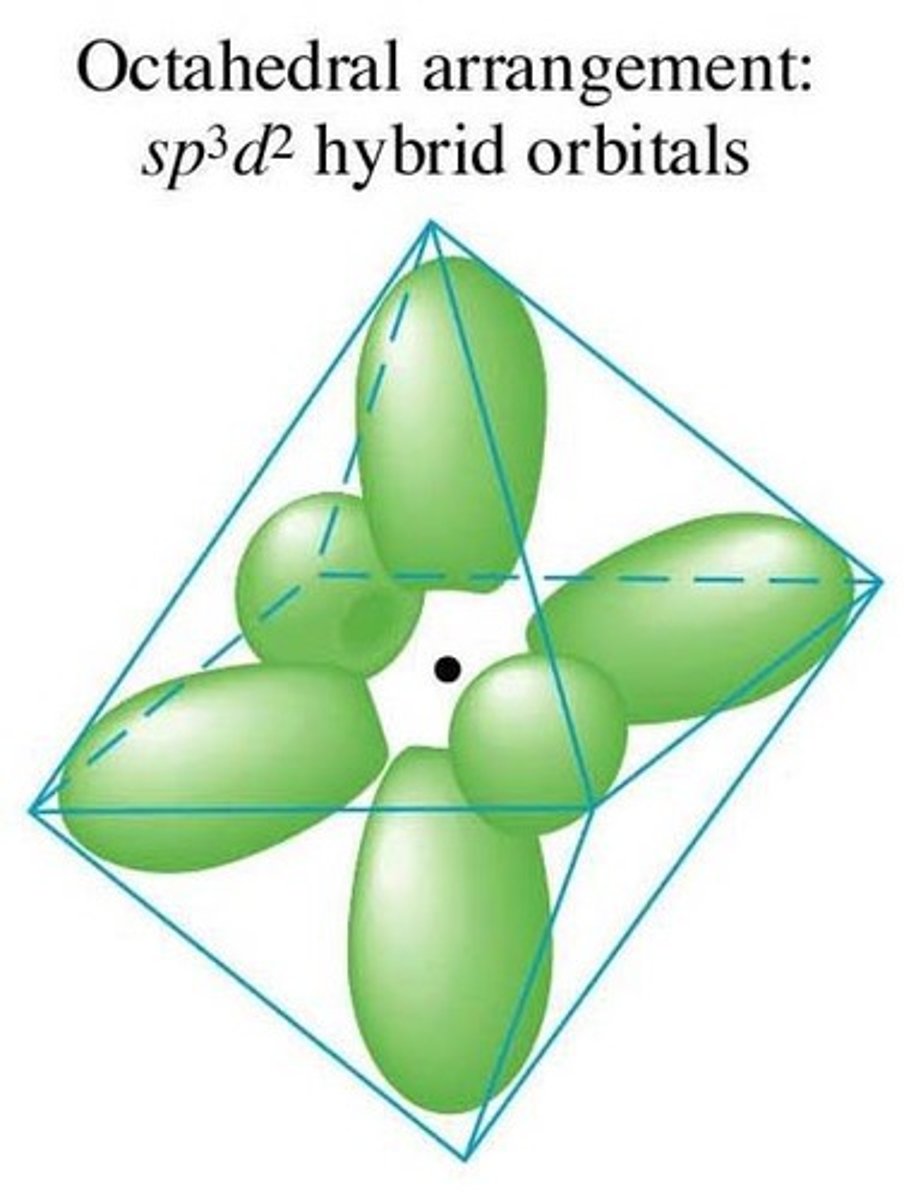
Octahedral
Electron group arrangement of 6 electron groups (AX6). Forms ideal bond angles of 90 degrees (Ex. SF6, IOF5)
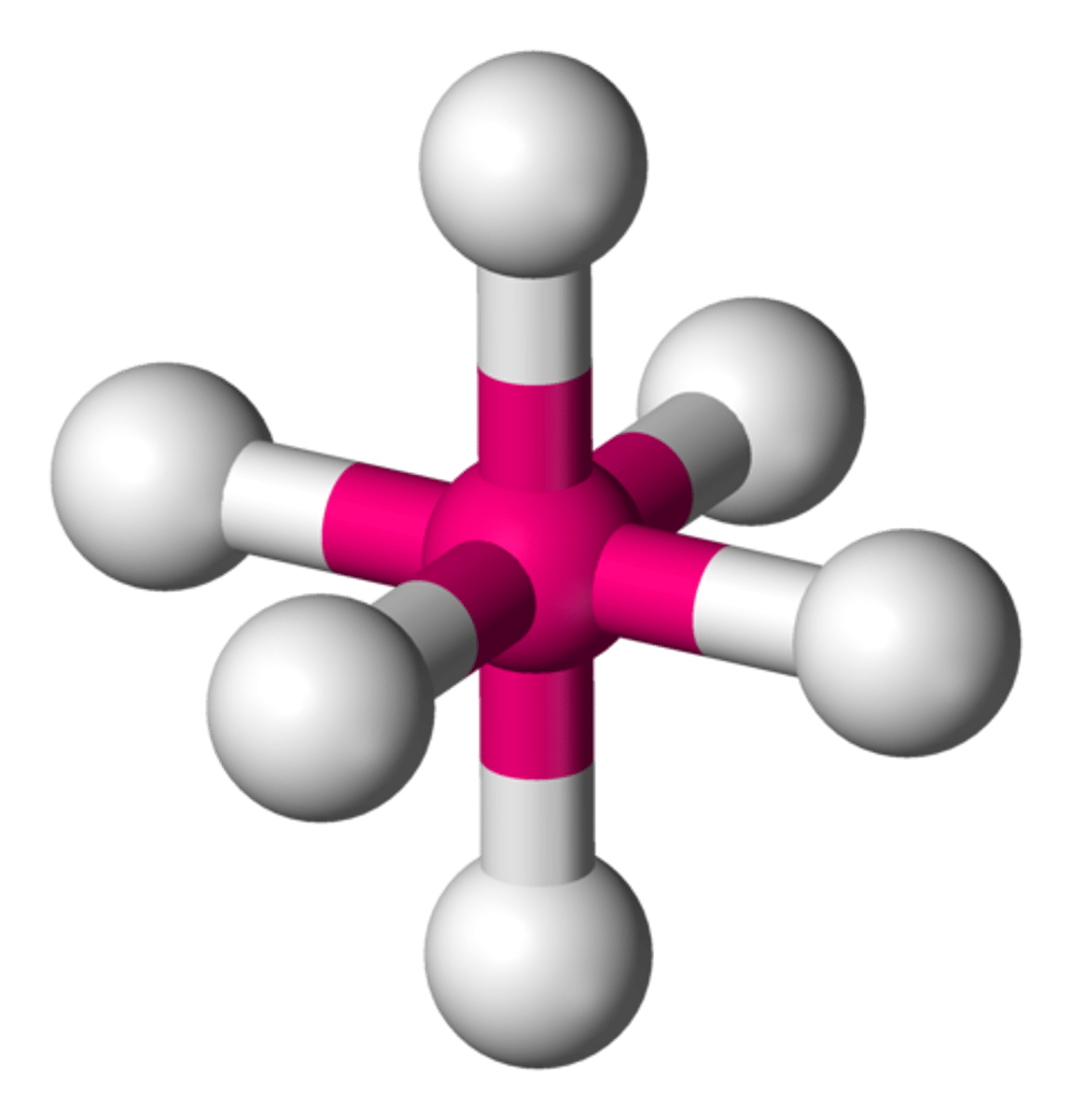
equal
If all of the electron groups around a central atom are bonding groups, then the electron group geometry will ____ the molecular geometry.

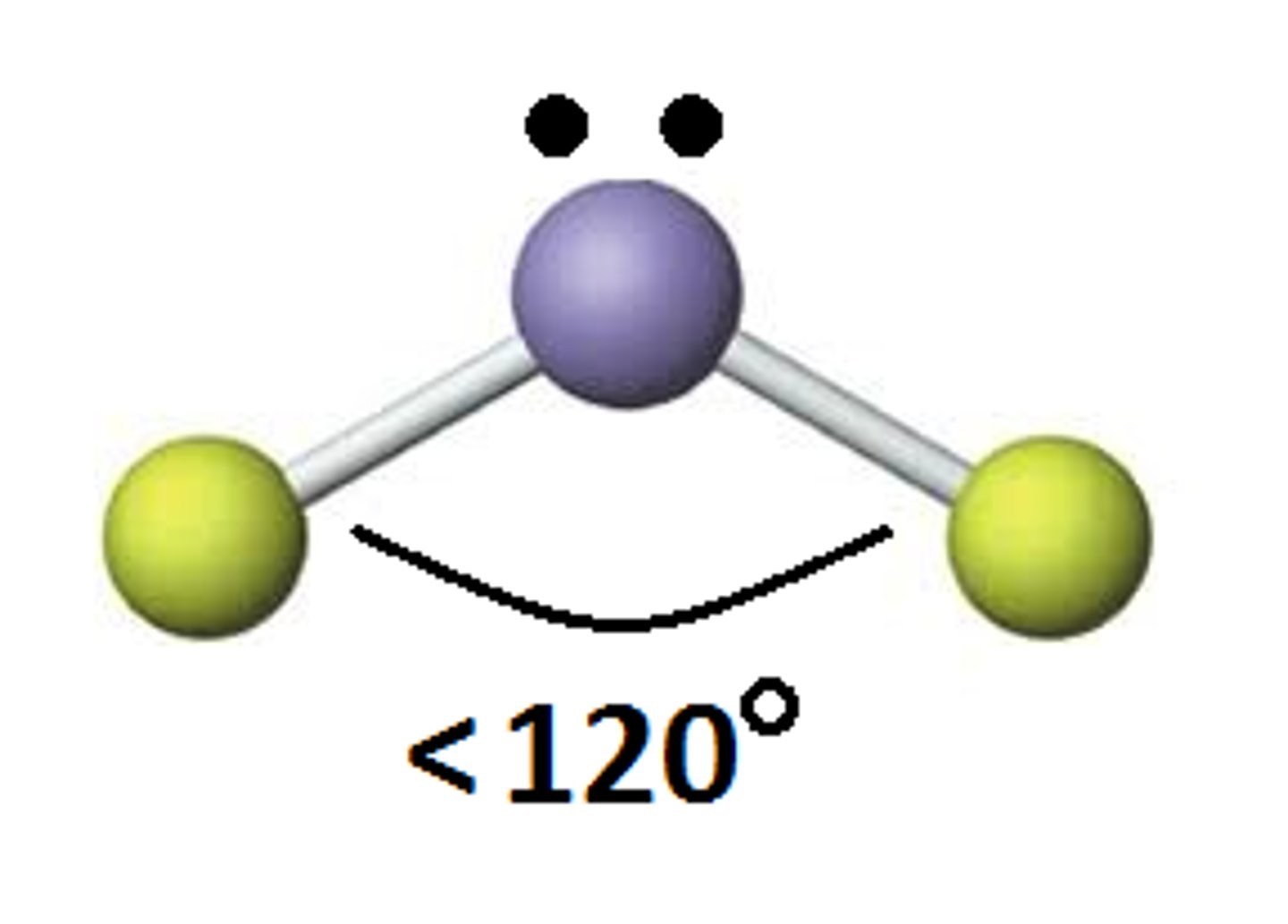
Bent
Molecular shape resulting from 2 bonding groups and 1 lone pair (AX2E). Forms bond angles of <120 degrees (Ex. SO2, O3)
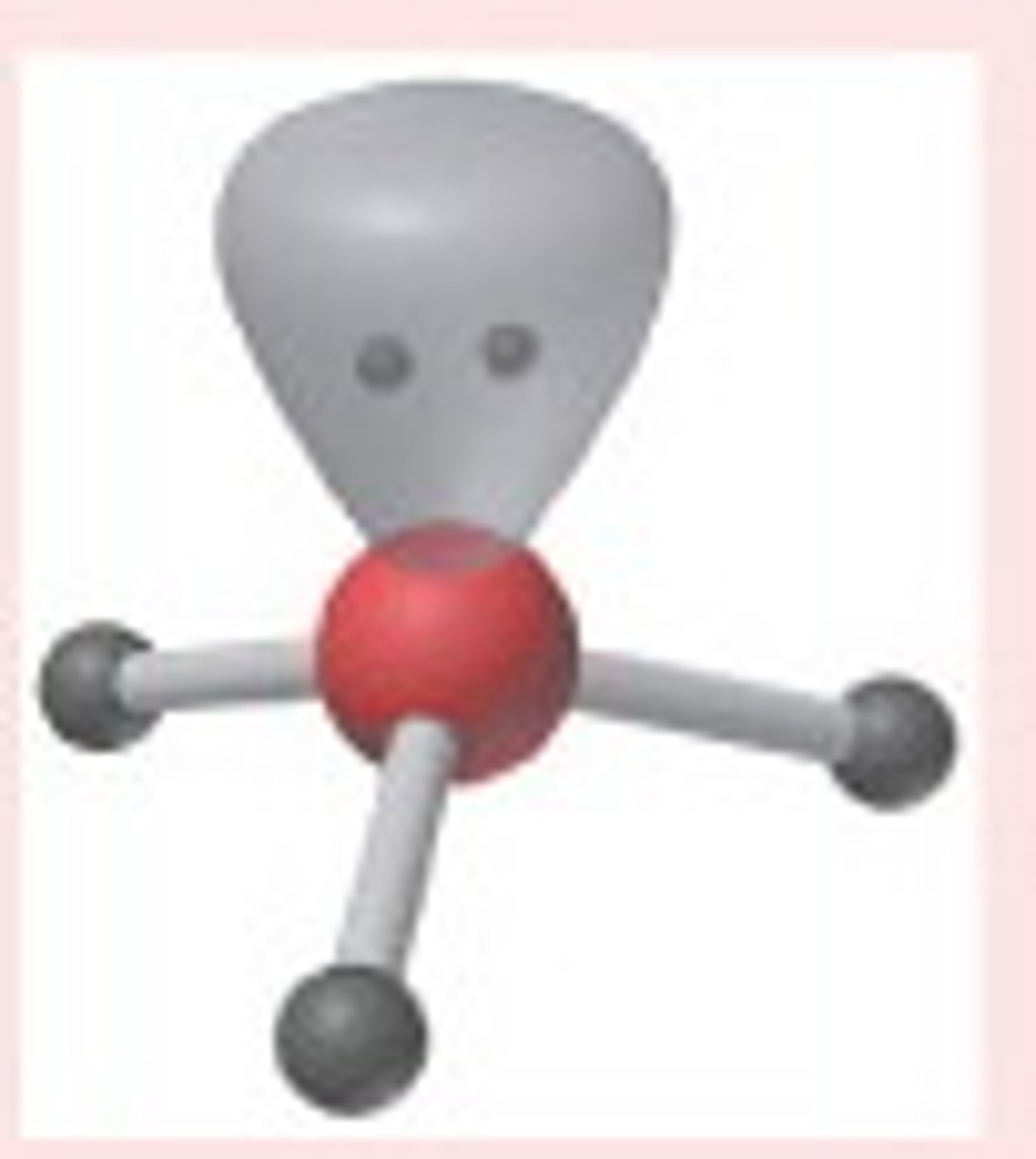
Trigonal Pyramidal
Molecular shape resulting from 3 bonding groups and 1 lone pair (AX3E). Forms bond angles of <109.5 degrees (Ex. NH3, PF3)
Bent
Molecular shape resulting from 2 bonding groups and 2 lone pairs (AX2E2). Forms bond angles of <109.5 degrees (Ex. H2O, OF2)
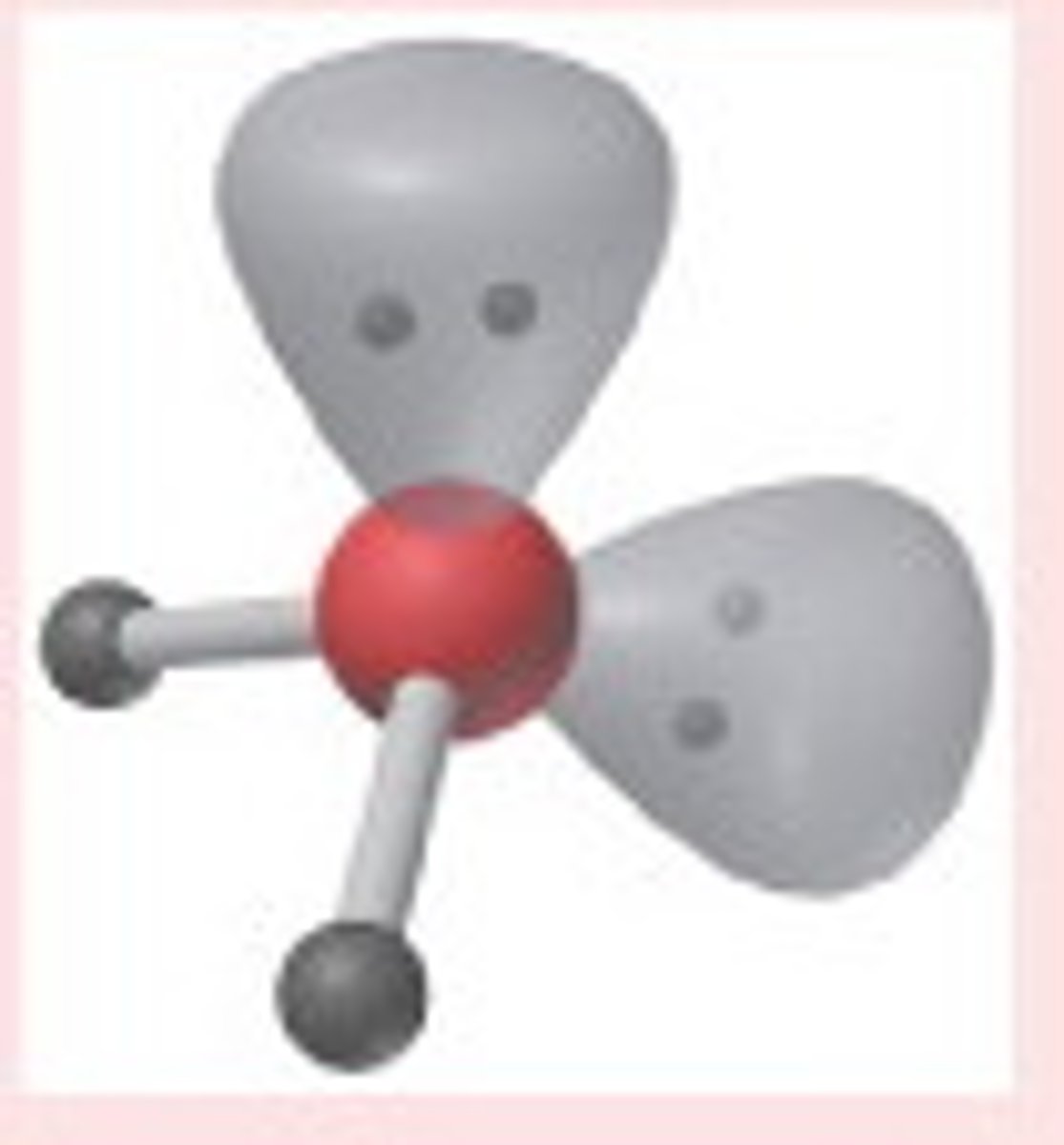
Seesaw
Molecular shape resulting from 4 bonding groups and 1 lone pair (AX4E). Forms bond angles of <120 degrees (equatorial) and <90 degrees (axial) (Ex. SF4, IF4)
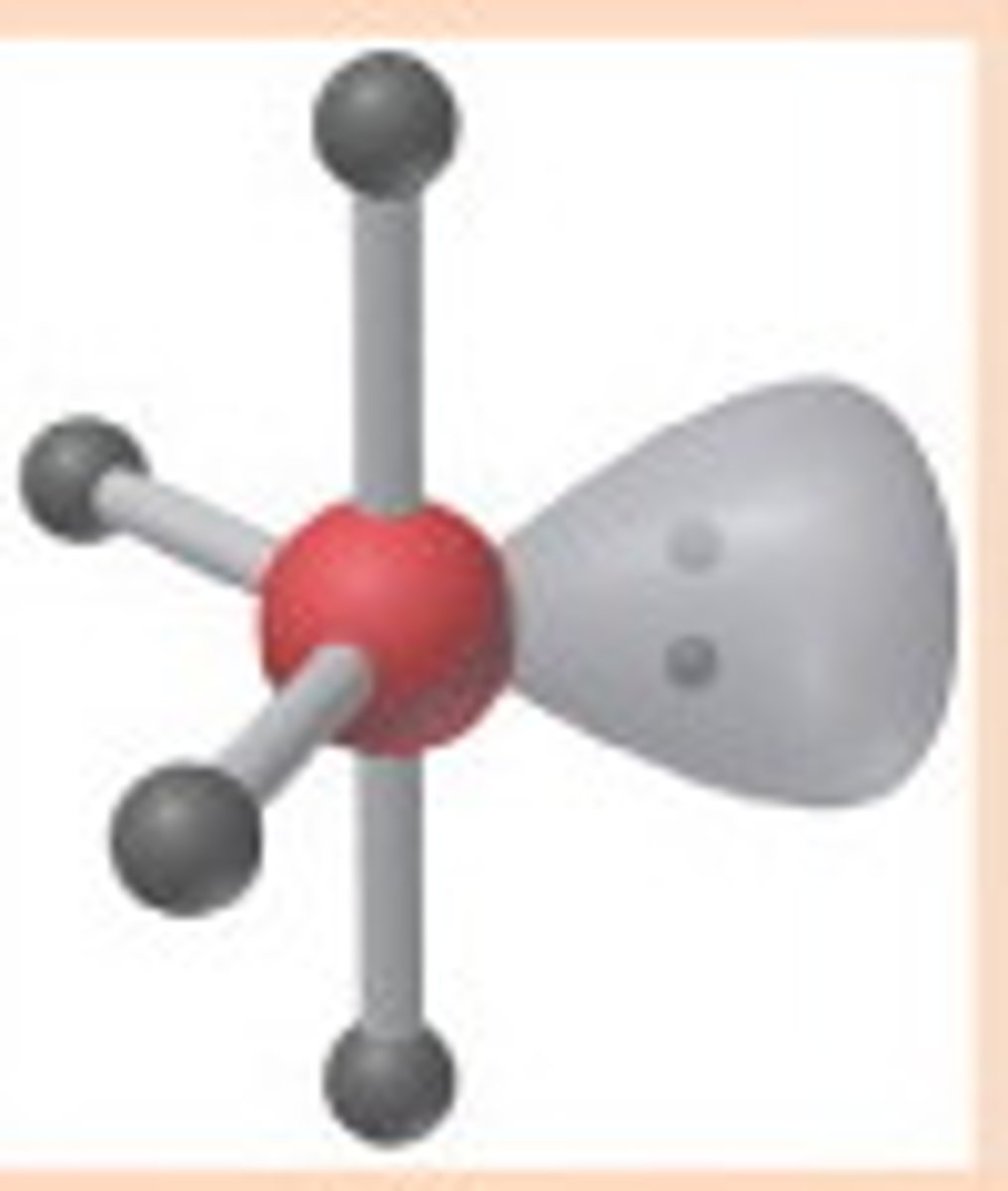
T-shaped
Molecular shape resulting from 3 bonding groups and 2 lone pairs (AX3E2). Forms bond angles of <120 degrees (equatorial) and <90 degrees (axial) (Ex. ClF3, BrF3)
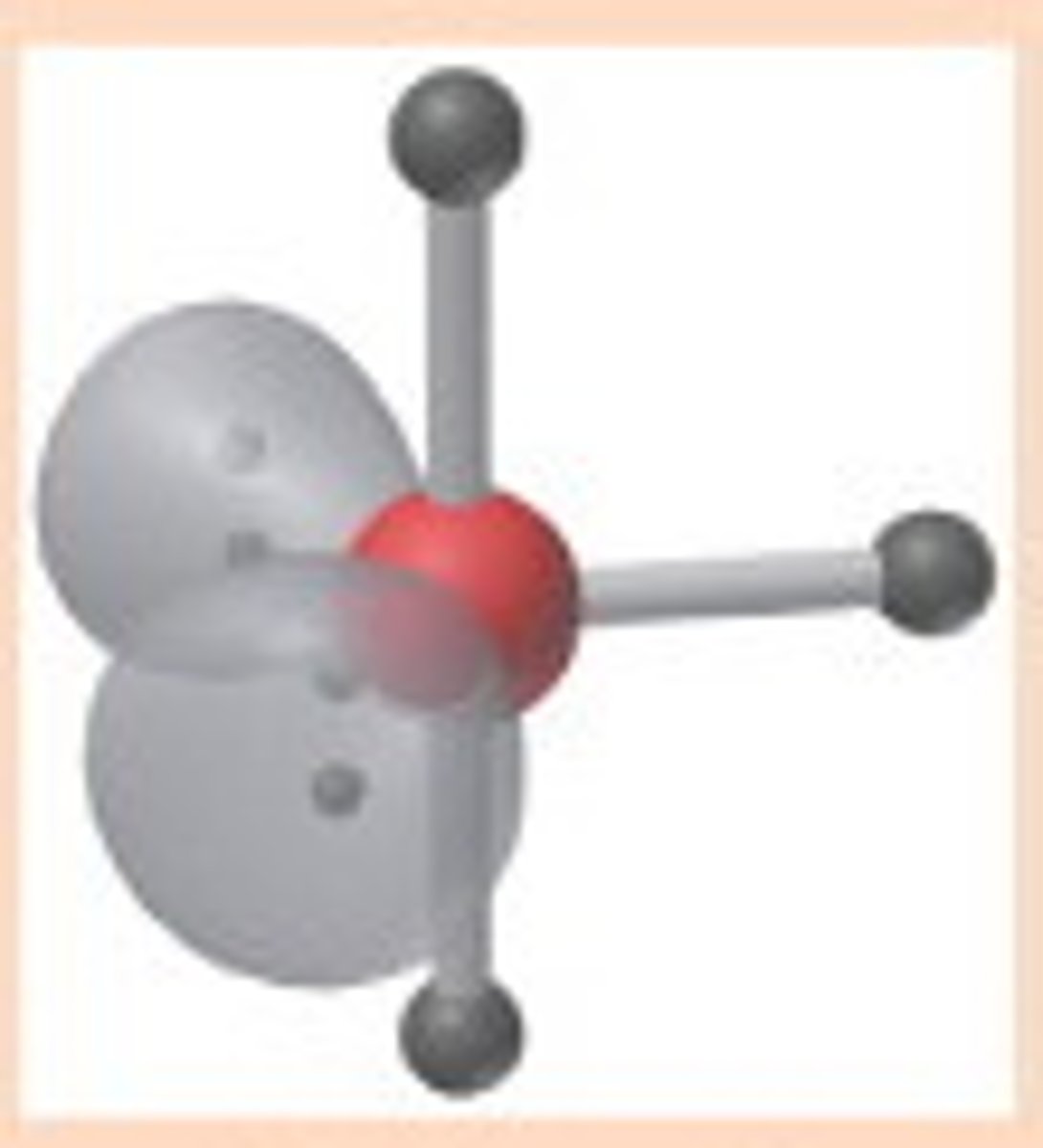
Linear
Molecular shape resulting from 2 bonding groups and 3 lone pairs (AX2E3). Forms bond angles of <120 degrees (equatorial) and 180 degrees (axial) (Ex. XeF2, I3)
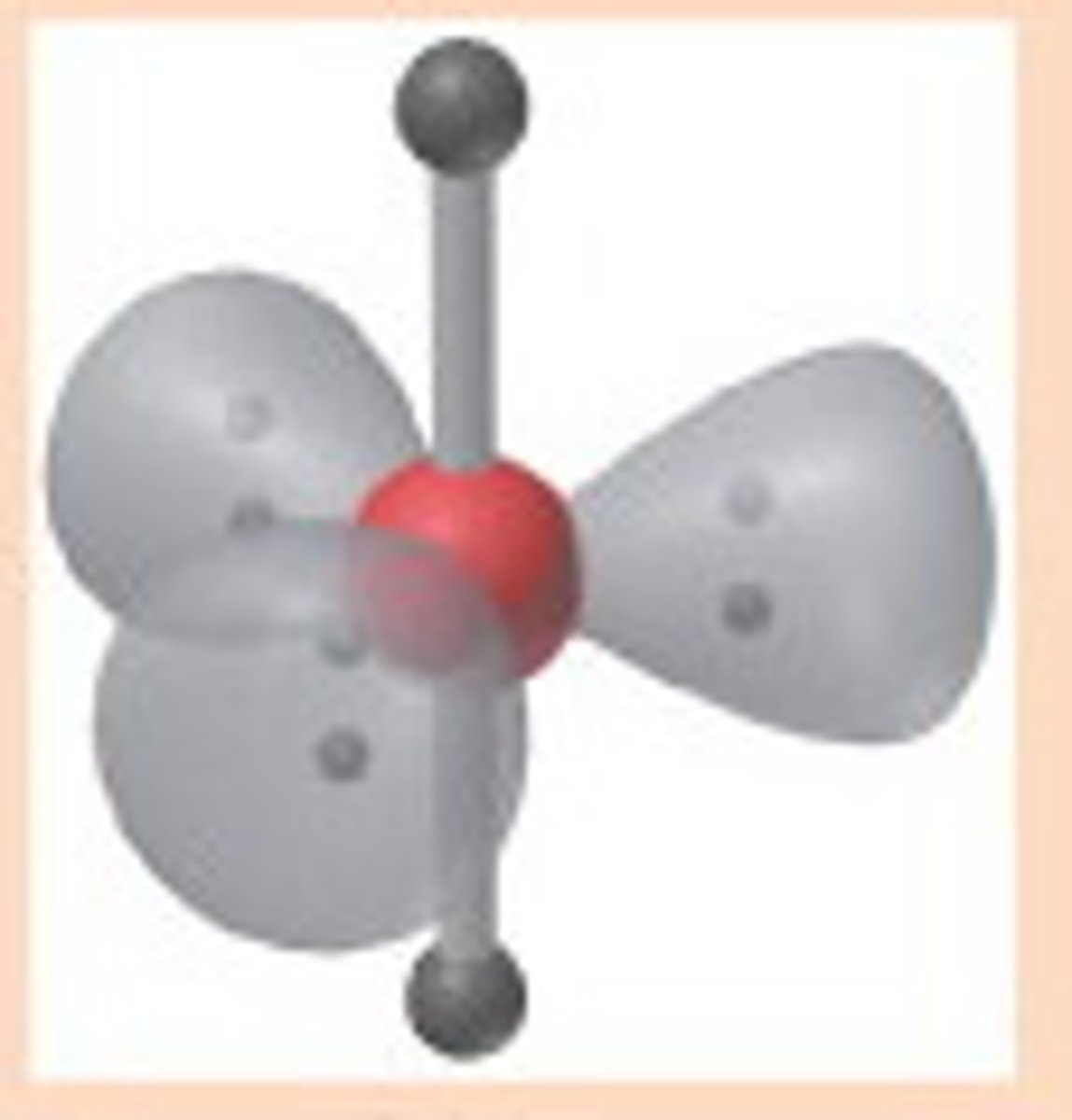
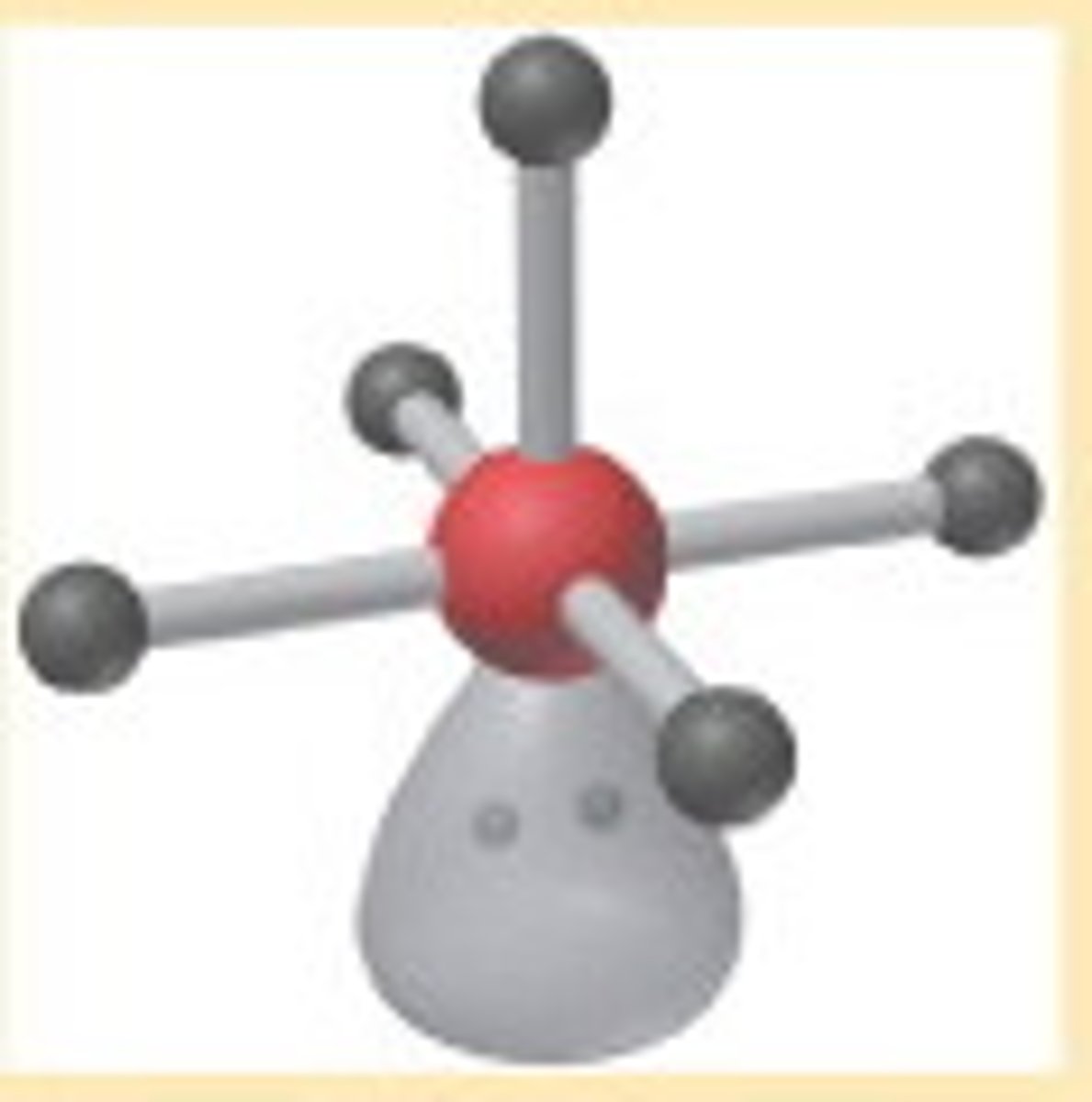
Square Pyramidal
Molecular shape resulting from 5 bonding groups and 1 lone pair (AX5E). Forms bond angles of <90 degrees (Ex. BrF5, XeOF4)
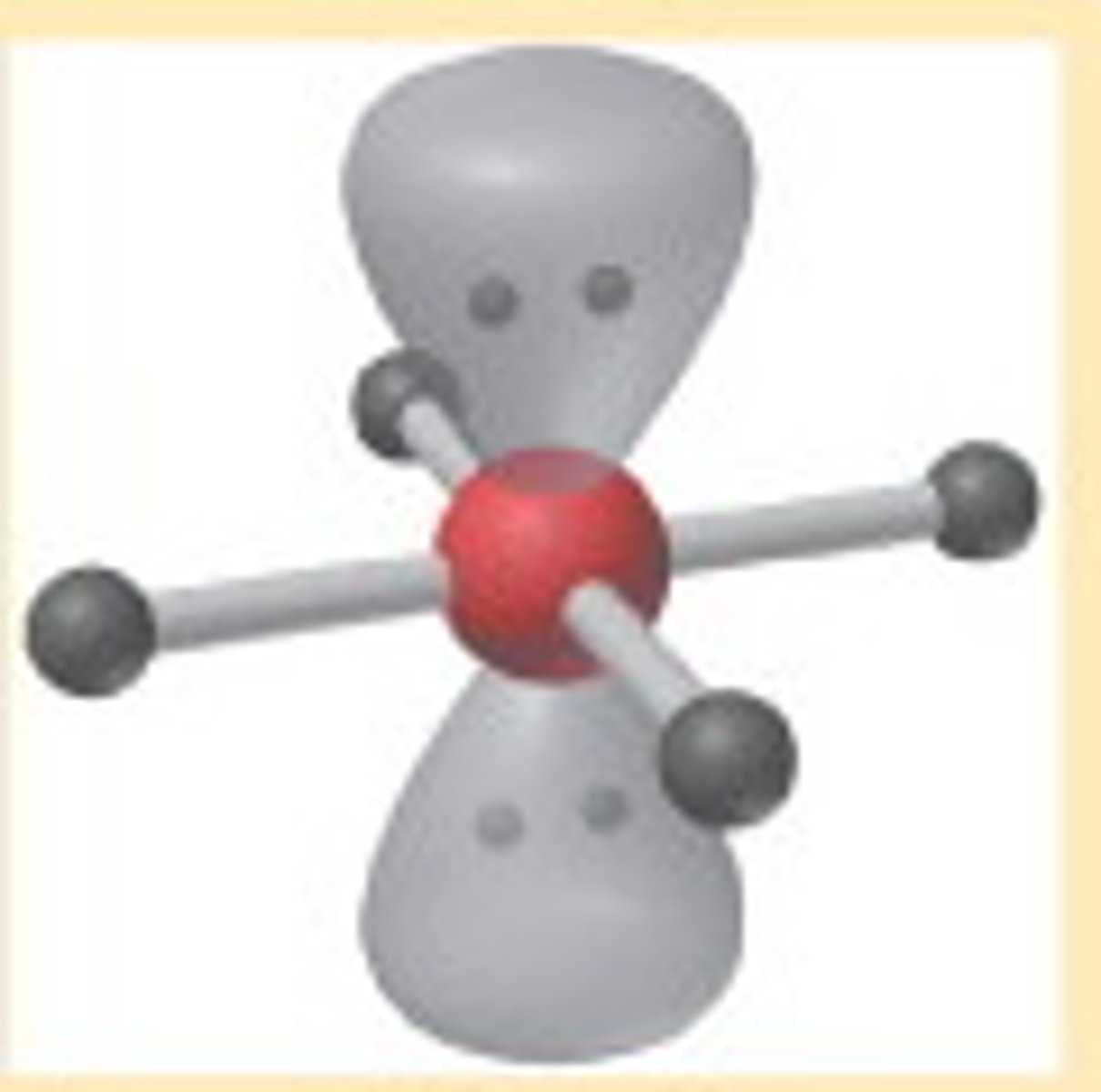
Square Planar
Molecular shape resulting from 4 bonding groups and 2 lone pairs (AX4E2). Forms bond angles of 90 degrees (Ex. XeF4, ICl4)
One s and one p
Two sp
Two p
For a hybridized molecule with linear electron geometry:
Which atomic orbitals are mixed?
Which hybrid orbitals are formed?
Which unhybridized orbitals are remaining?

One s and two p
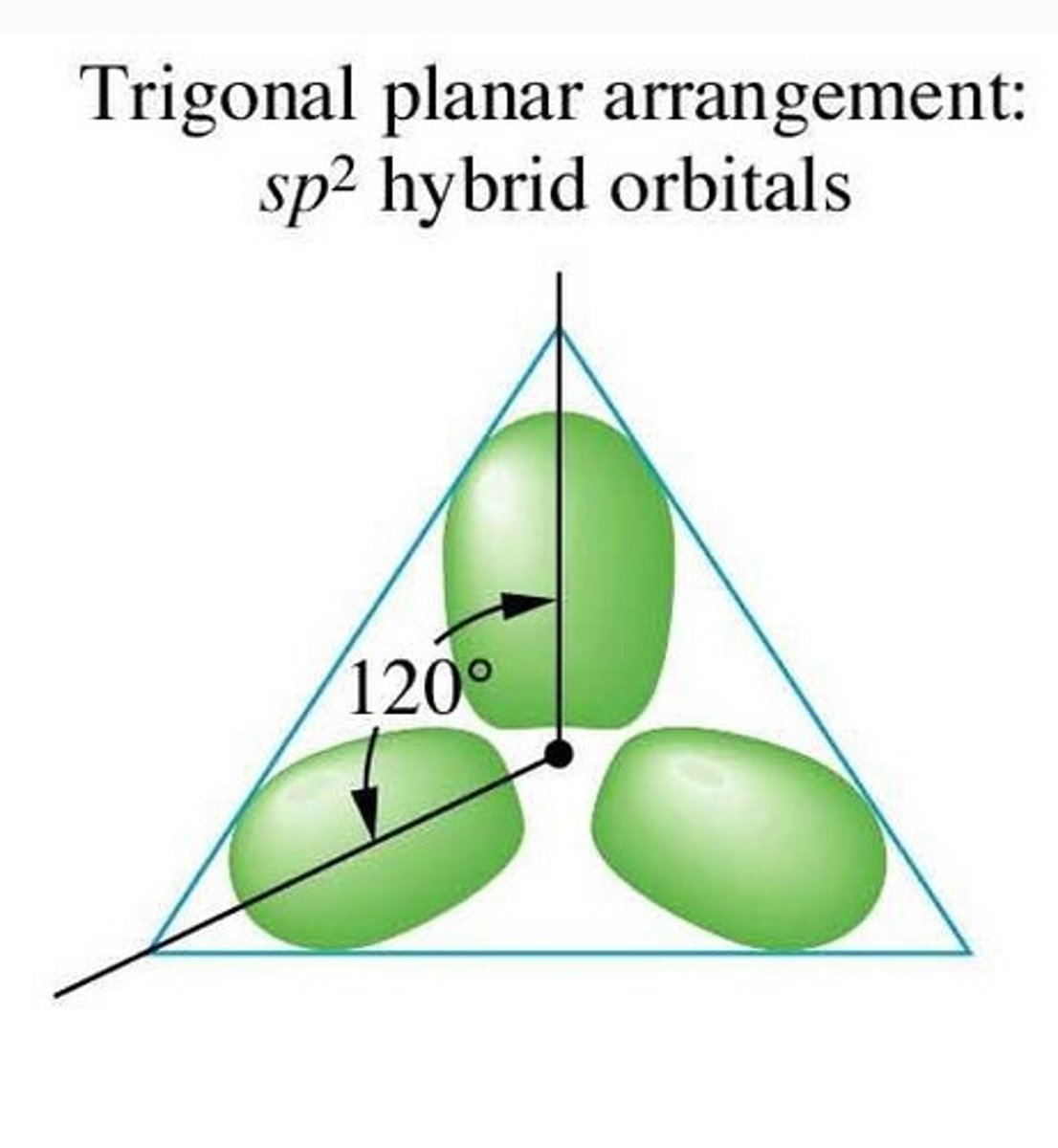
Three sp2
One p
For a hybridized molecule with trigonal planar electron geometry:
Which atomic orbitals are mixed?
Which hybrid orbitals are formed?
Which unhybridized orbitals are remaining?
One s and three p
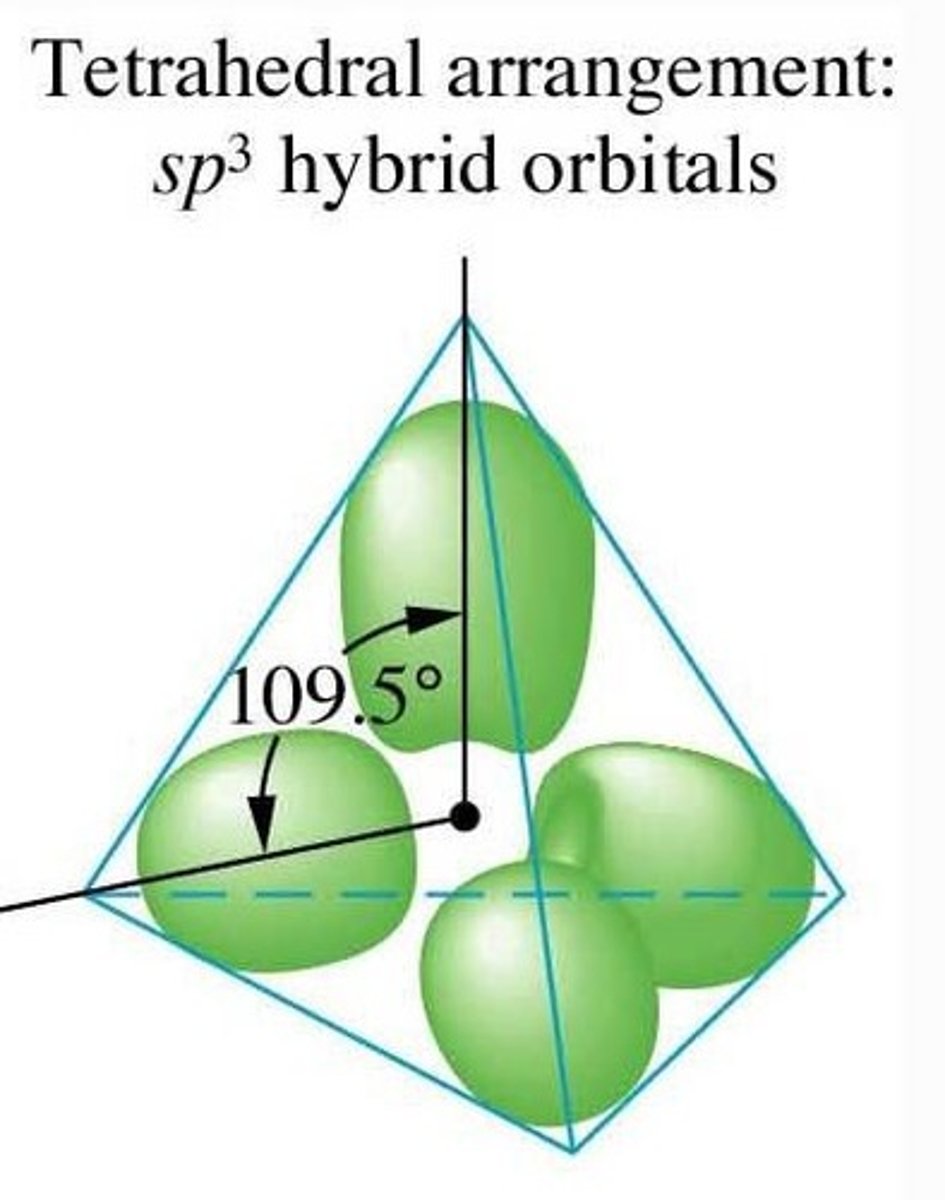
Four sp3
No p remaining
For a hybridized molecule with tetrahedral electron geometry:
Which atomic orbitals are mixed?
Which hybrid orbitals are formed?
Which unhybridized orbitals are remaining?
One s, three p, and one d
Five sp3d
Four d remaining
For a hybridized molecule with trigonal bipyramidal electron geometry:
Which atomic orbitals are mixed?
Which hybrid orbitals are formed?
Which unhybridized orbitals are remaining?
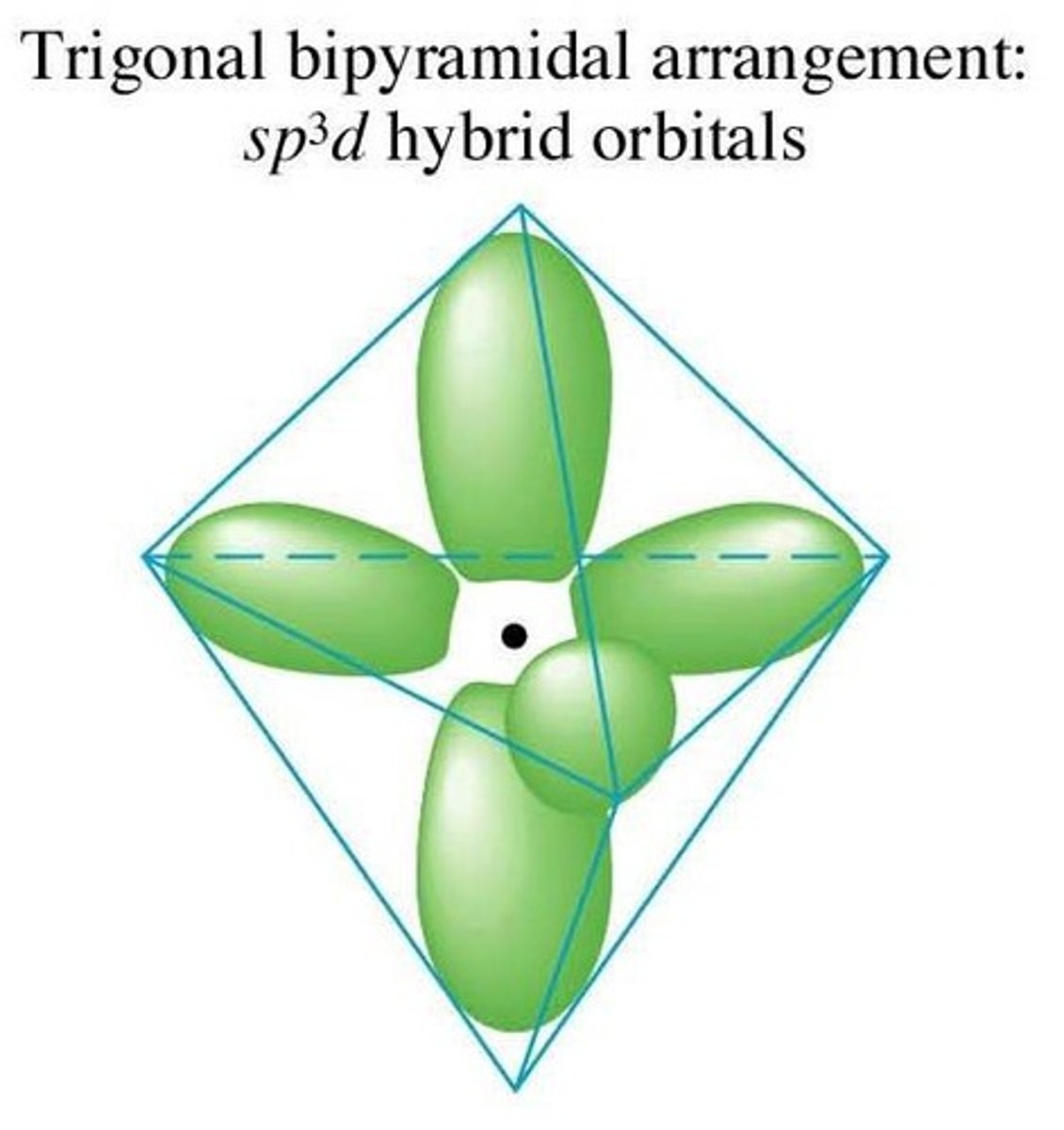
One s, three p, and two d
Six sp3d2
Three d remaining
For a hybridized molecule with octahedral electron geometry:
Which atomic orbitals are mixed?
Which hybrid orbitals are formed?
Which unhybridized orbitals are remaining?