05A Protein Import [ Intro to cytosol ]
1/86
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
87 Terms
This organelle contains a little more than half of the total cell volume
Cytosol
What are the components of the cytoplasm
Cytosol and Cytoplasmic organelles
Where protein synthesis and degradation occurs
Cytosol
True or false: Intermediary metabolism occurs in the cytosol ( catabolic and anabolic reaction s )
True
What do you call the sustem of organelles that have topologically similar membranes or have similar structures
The endomembrane system
What are included in the endomembrane system or organelles that are membrane bounded compartments, with distinct functions due to the specialized mix of protein content
Er
GA
Lyso
Endosomes
Peroxi
Mito and plastids
This organelle is where the synthesis and modification of lipids and proteins for distribution occur
Endoplasmic reticulum
This organelle is where modification, sorting, and packaging of proteins for delivery occur
Golgi Apparatus
This organelle is where intracellular degradation occurs
Lysosomes
Organelle that facilitates the sorting of endocytosed material
Endosomes:
Organelle that performs various oxidative reactions
Peroxisomes
What are the different Protein Transport Mechanisms [ And their directionality ]
a. through pores or Gated Transport [ Nuclear Pore Complexes ] - bidirectional
b. transport across membranes [ Transmembrane protein translocators ] - unidirectional
c. transport by vesicle - bidirectional
What organelles do transport via pores or gates transport
Nucleus via Nuclear Pore Complexes
What organelles do transport via transmembrane protein translocators
Chloroplast
Mitochondria
Peroxisomes
What organelles do transport via vesicles
Endoplasmic Reticulum
Golgi Apparatus
Lysosomes
Endosomes
Directionality of Protein Sorting
Unidirectional
What defines a protein’s fate in protein sorting
Amino acid sequences
General Requirements for Protein Transport
Recognition signals or Signal sequences
Energy
Receptors
Translocators
These are specific amino acid sequences that gives the address where the protein needs to go or direct proteins to different locations
recognition signals or signal sequences
general term and other terms for recognition signals or signal sequences
General Term: protein sorting signal sequences
Other term: signal peptide/signal sequence/targeting sequence
What are the two types of recognition signals or signal sequences
Terminally located [ amino or carboxyl end ]
Internally Located
What enzyme usually cleaves Terminally located signal peptides or recognition signals
signal peptidase - FOR TERMINALLY LOCATED OKAY
*internal located are not usually cleaved
For terminally located signal sequences, how long are the continuous stretch of amino acids at the amino end of the protein to be sorted
15-60 [ THIS IS FOR THE AMINO END OKAY ]
This kind of signal sequence may be made up of patches that fold into specific 3D arrangement
Internally Located
Once a terminally located signal sequences gets folded, describe the orientation of these specific sequences
once it is folded, the signal sequence is located at the surface of the protein, so that it can easily bind with the receptor
Once the internally locates signal sequence gets folded, what is formed
Signal patch gets formed [ hydrophobic regions ] that exhibits molecular complementarity with a receptor
signal sequence for nucleus, perozisome, return to the ER
-KKKRK-
-SKL-COO-
-KDEL-COO
This requirement for protein transport powers the transport from different sources [ and what are these different sources ]
Energy
ATP, GTP,
Proton Motive Force
Or can be a spontaneous reaction
these protein transport requirement have molecular complementarity with the signal sequences
Receptors
these protein transport requirement are for recognition by other proteins that guides the proteins to their destination. These are either located on the cytosol or on the organelle membrane
Receptors
surface amino acids of protein are recognized by receptors to have stable binding based on what
chemical properties (molecular complementarity)
True or False: Receptor binding Recognize classes of proteins rather than individual protein species
True
Fate of receptors after unloading the cargo
They are recycle or reused
These are proteins that facilitate the transport that are embedded in an organelle membrane [ what are the functions as well ]
Translocators
Functions: for protein binding [ it allows also the integration of the protein into the membrane itself] and transport assistance
These protein sorting requirement can be modeled using thermodynamics and kinetics
Translocators
True or false: Protein import can be co-translational or post-translational
True
For post-translational import, protein synthesis occurs where
Free ribosomes in the cytosol that would get imported inside organelles [ Nucleus, Mito, Plastid, Peroxi ]
For co-translational import, protein synthesis occurs where
proteins synthesis occurs by being docked on the Rough ER ( translation and transcription are coupled )
This can then remain in the ER or go to GA and gets transported to the other organelles
Requirements for Nuclear Import
Signal Sequences: NLS - Nuclear Localization Signals
Energy: Hydrolysis of GTP via GTPase RAN (abundant in the cytoplasm)
Translocators: NPC
Receptors: Nuclear Transport Receptors [ Importin ]
Delivery sites for the nucleus
nuclear envelope
nucleoplasm
nucleus
the signal sequences for Nucleus import or the NLS [ Nuclear localization sequences ] are rich in what amino acids
Lysine (K) and Arginine (R) which are + charged AA
What is the Receptor for the import of proteins inside the nucleus called
Nuclear Transport Receptor - Importin
Explain the study that confirmed the role of NLS in protein import in this immunofluorescence micrographs
The normal antigen/signal sequence which is rich in lysine was successfully imported into the nucleus (A). When lysine was replaced by threonine, the T-antigen stayed in the cytosol as represented by immunofluorescence
What is the requirement for nuclear Export
Signal Sequences: NES - nuclear export signals (NES)
Energy: Hydrolysis of GTP via GTPase RAN (abundant in the cytoplasm)
Translocators: NPC
Receptors: Nuclear Transport Receptors [ Exportin ]
the signal sequences for Nucleus Export or the NES or Nuclear export signals are rich in what amino acids
leucine [ The Classic NES ]
What are the different processes that happens inside the nucleus that it is important to have protein import into the nucleus
ribosomal unit assembly
protein transcription
proteins for replication
proteins for chromosome packing
These act as an importin and exportin for different cargos in the Simultaneous import and export of proteins and it s examples
Bidirectional Karyopherins [ Importin 13, Exportin 1, Exportin 5 ]
True or false: In the SIMULTANEOUS IMPORT AND EXPORT OF PROTEINS, Cleaving is ABSENT (why)
True, Cleaving is absent for the NLS and NES since Importin and Exportin utilizes these as binding sites
This is the largest protein complex in the cell, that is made up of polypeptide subunits lining a pore in the nuclear envelope that are for import and export in the nucleus
Nuclear pore complex (NPC)
These components serves as protection during the exchange of components between nucleus and cytoplasm, or simply during bidirectional traffic.
Nuclear pore complex (NPC)
How does bidirectional Traffic occur in the nucleus and cytosol
Nucleus needs proteins imported from the cytoplasm, while RNA transcripts from the nucleus are exported to the cytoplasm [ During exchange or products ]
serve as a gate to regulate or prevent entry of materials through the nuclear envelope
Nuclear pore complex (NPC)
What are the parts of the Nuclear pore complex (NPC)
a. transporter ( the actual pore )
b. spokes ( attached to the anchor protein )
c. anchor proteins ( anchors the entire NPC to the membrane of the nuclear pore )
d. ring subunits ( where fibers are attached )
e. fibers ( faces the cytosol ) and Basket fibers ( faces the nucleoplasm ) - directly interacts with the protein so that it can determine whether if not it can pass thorough
specific part of the Nuclear pore complex (NPC) that directly interacts with the protein so that it can determine whether if not it can pass thorough
fibers
These are nuclear transport receptor protein (karyopherin) for import that binds NLS
Importin
Explain the Nuclear import of proteins
Importin + NLS of a cargo protein = Importin-NLS containing Cargo Complex
Goes through the NPC via diffusion with FG-nucleoporins, entering the nucleoplasm
RAN-GTP + Importin Cargo Complex [ Causing conformational change ] = RAN-GTP-Importin + Cargo Protein
RAN-GTP-Importin Exits the nucleoplasm via NPC
in the NPC there are cytoplasmic filaments with GTPase Activating Protein
Cytosolic side GTP Hydrolysis: RAN-GTP-Importin + GAP or GTPase Activating Protein = RAN-GDP + Importin
importin gets ready for another round
Ran-GDP is returned to the nucleoplasm + GEF = Ran-GTP
Explain the Nuclear Export of proteins
Nucleoplasmic side: Exportin 1-NES containing Cargo protein-Ran GTP complex
diffusises to the NPC via transient interaction with FG nucleoporins
GTP Hydrolysis in the cytosolic side
Cargo protein gets released and remains in the cytosol
Exportin 1 - recycled in the nucleoplasm
Ran-GDP + GEF ( in the nucleoplasm) = Ran GTP
Explain the RAN-Independent Export of mRNAs
Heterodimeric NXF1/NXT1 complex binds to mRNA protein (nucleoplasmic side)
NPC via transient interaction with FG- nucleoporins
mRNA e- erodimer NXF1/NXT1 —RNA helicase Dbp5—> NXF1/NXT1 _ mRNA
NXF1/NXT1 is recycled back to the nucleus by RAN dependent process
Ran-GDP is converted back to Ran-GTP via
Ran- GEF or Ran Guanine Nucleotide Exchange Factor
meaning of mRNPs
mRNA prtotein complexes [ complex of heterodimeric NXF1/NXT1 and mRNA ]
What enzyme facilitates the hydrolysis of complex of heterodimeric NXF1/NXT1 and mRNA
RNA helicase Dbp5
What causes the hydrolysis of RAN GTP when being transported to the cytosolic side via NPC
GTPase activating protein located at the cytoplasmic filaments of the NPC
Requirements for Nucleolus Transport
signal sequences: NOS or nucleolar targeting sequence
Energy : Hydrolysis of GTP via GTPase
receptor
translocator: NPC
what are the amino acids comprising of the signal sequences: NOS or nucleolar targeting sequence for the transport in the nucleolus
basic amino acids (argenine and lysine rich)
True or false: Cleaving is also absent in NOS just like NLS and NES as they serve as binding sited allowing the entry and exit of the molecules
True
What are usually transported in the nucleolus
ribosomal proteins since transcription of rRNA occurs here, the rDNA LOCUS consist of different genes that codes for genes in the rna product
This is a process by which ribosomes are produced, and what proteins are responsible for this process
Ribosomal biogenesis
Ribosomal proteins
nucleolar substructure
Explain the process of Ribosomal biogenesis
i. Transcription of rRNA (small and large subunits) and RNA transcripts for the ribosomal proteins
ii. Translation of ribosomal proteins by cytosolic ribosomes
iii. Import of ribosomal proteins into nucleolus
iv. Cleavage and modification of rRNA (small and large subunits)
v. Assembly of small and large ribosomal subunits (in nucleus)
vi. Export of small and large ribosomal into cytosol and assembly
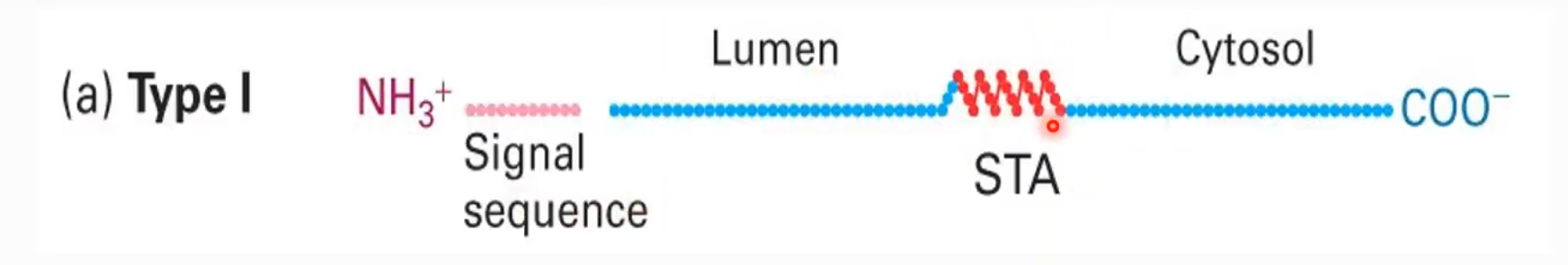
Requirements for Cytosolic Transport
Signal sequence: Various ( no specific bec. of varying materials being transported)
Energy: Hydrolysis of GTP via GTPase - nucleus to cytosol only and others are ATP, PMF or spontaneous - depending on the organelles
Translocator
Receptor
Describe teh cleaving conditions of cytosol
cleaving will depend on the protein to be transported
FATES of Cytosolic proteins
binding to the plasma membrane
covalent modification
rapid degradation if misfolded (undergoes ubiquitylation)
Where does protein covalent modification happen ?
In the cytosol
In protein covalent modification, what do you call the process of attaching the +CHOS
Protein glycosylation
In protein covalent modification, what is an example of a coenzyme you attach ?
Biotin
In protein covalent modification, what do you call the enzyme that attaches a methyl group to the protein
Methyl transferases
In protein covalent modification, what do you call the enzyme that attaches a phosphate group to the protein
Kinases ( reversible - regulating the activity of the enzyme )
What do you call the enzyme that attaches an acetyl group during protein covalent modification ?
Acetyl carboxylase and acetylase - reversible
These are proteins that are for degradation, carrying degredation signals. It degrades by carrying destabilizing amino acids
Degrons
Memorize the destabilizing and stabilizing amino acids
Okay
What enzymes cause direct transfer of ubiquitin to the substrate
E2 and E3
What happens during conjugation in ubiquitin proteosome pathway
E3-substrate complex binds to E2, ubiquitin is theN transferred from E3 to substrate, E2E3 then dettaches
Multiple rounds of ubiquinylation occur, with ubiquitin attaching to one of the Lysines
Polyubiquitylation
To what specific part of the substrate will ubiquitin bind to during polyubiquitylation
Lysine ( k48m for proteins targeted for degradation ) on the previous ubiquitin
What is the enzyme that does protein degradation after polyubiquitylation
26S proteosome
Steps during UBIQUITIN-PROTEOSOME PATHWAYS
Activation via E1
Transfer via E2
Ligation via E3
Conjugation
Polyubiquitylation
Deubiquitylation and degradation via 26s proteosome
A macromolecular complex with multi catalytic proteinase activity for the breakdown of ubiquitinated proteins
26s Proteosomes
Parts of the 26s proteosomes
2- 19s regulatory particle
1 - 20s proteosome [ with Alpha and beta sub units ]