Rules for Oxidation States
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
4 Terms
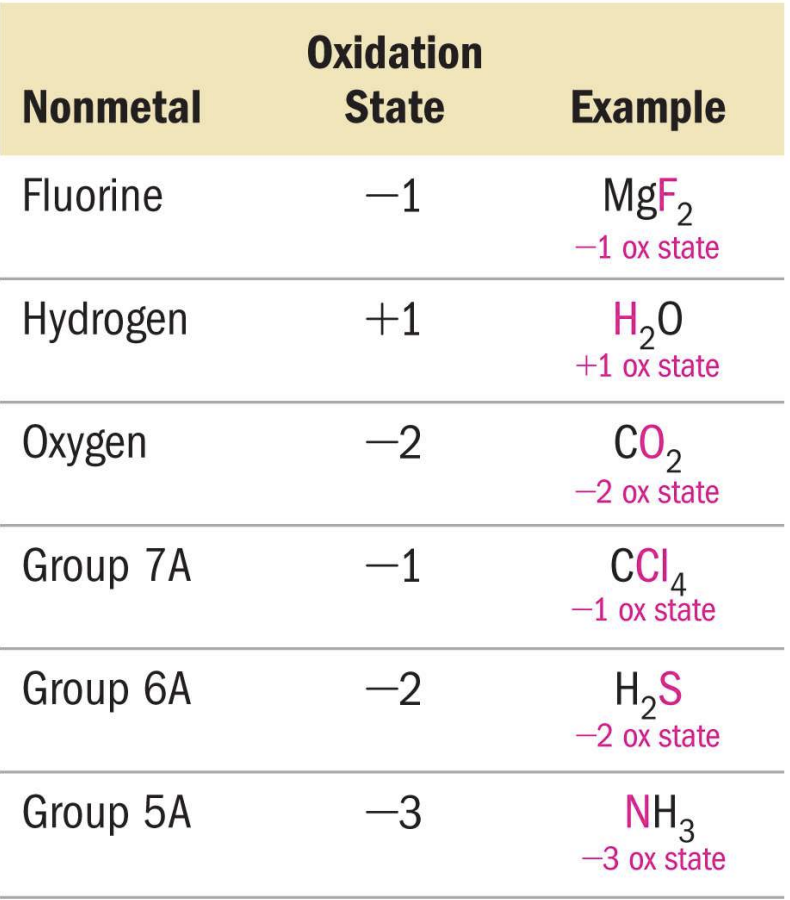
Rules for Oxidation States.
1.) Oxidation state of pure elements is 0
2.) Oxidation state of mono-atomic ion is equal to its charge
3.) Sum of oxidation in a chemical species equals its charge
4.) Oxidation states of metals are always positive (group 1A metals are always +1, and group 2A metals are always +2)
5.) Use the table above, with entries at the top of the table being more important than entries at the bottom of the table
Rules for balancing Redox Reaction under acidic conditions
1.) Separate into half-reactions
2.) Balance elements other than O and H
3.) Balance O by adding H2O
4.) Balance H by adding H+
5.) Balance net charge by adding e-
6.) Combine and balance electrons.
Rules for balancing Redox reactions under Basic conditions
1.) Separate into half-reactions
2.) Balance elements other than O and H
3.) Balance O by adding H2O
4.) Balance H by adding H+
5.) Balance net charge by adding e-
6.) Combine and balance electrons.
7.) Add OH- for every H+ to both sides
Skip
Skip