Secondary
0.0(0)
Card Sorting
1/5
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
1
New cards
Process
Discharge results in spontaneous cell reactions, while recharge results in non-spontaneous reactions.
Anode (negative) is the place of oxidation, cathode (positive) is the place of reduction.
Both recharge and discharge involve electron flow from anode to cathode.
Recharge electrode becomes cathode, and a voltage greater than discharge voltage is applied to overcome internal resistance.
2
New cards
What is it
Stores its reactants but can be recharged
3
New cards
Advantages
Rechargeable, long useful life, cheaper
4
New cards
Disadvantages
Toxic lead, corrosive sulfuric acid, low energy density
5
New cards
Draw one
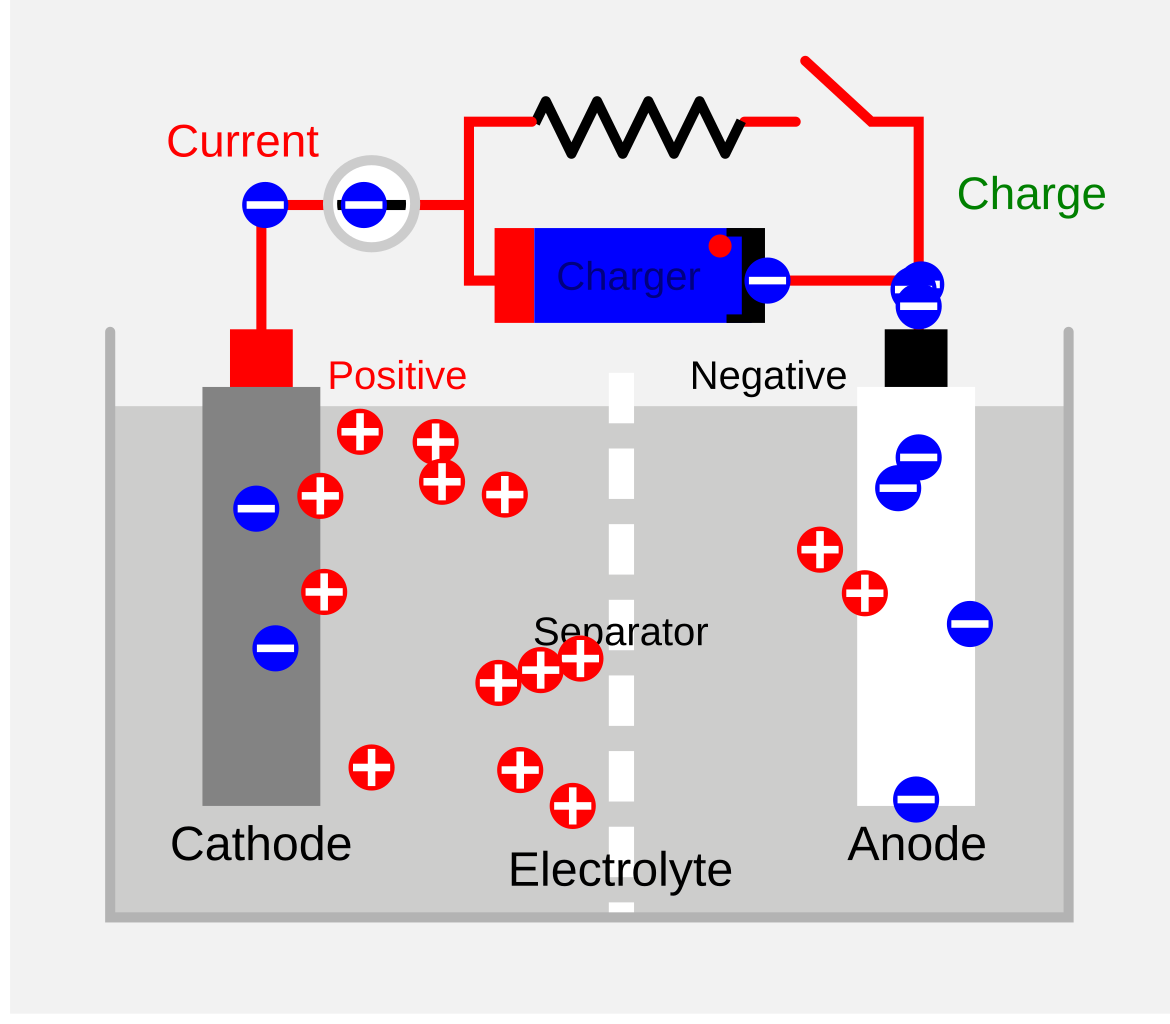
6
New cards
E˚
2.05V