Theories of Covalent Bonding and Hybridization
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
Molecular Orbital Theory
Bonding electrons reside in molecular orbitals from atomic orbitals.
Valence Bond Theory
Valence electrons are involved in chemical bonding.
Hybridization
Mixing of atomic orbitals to form hybrid orbitals.
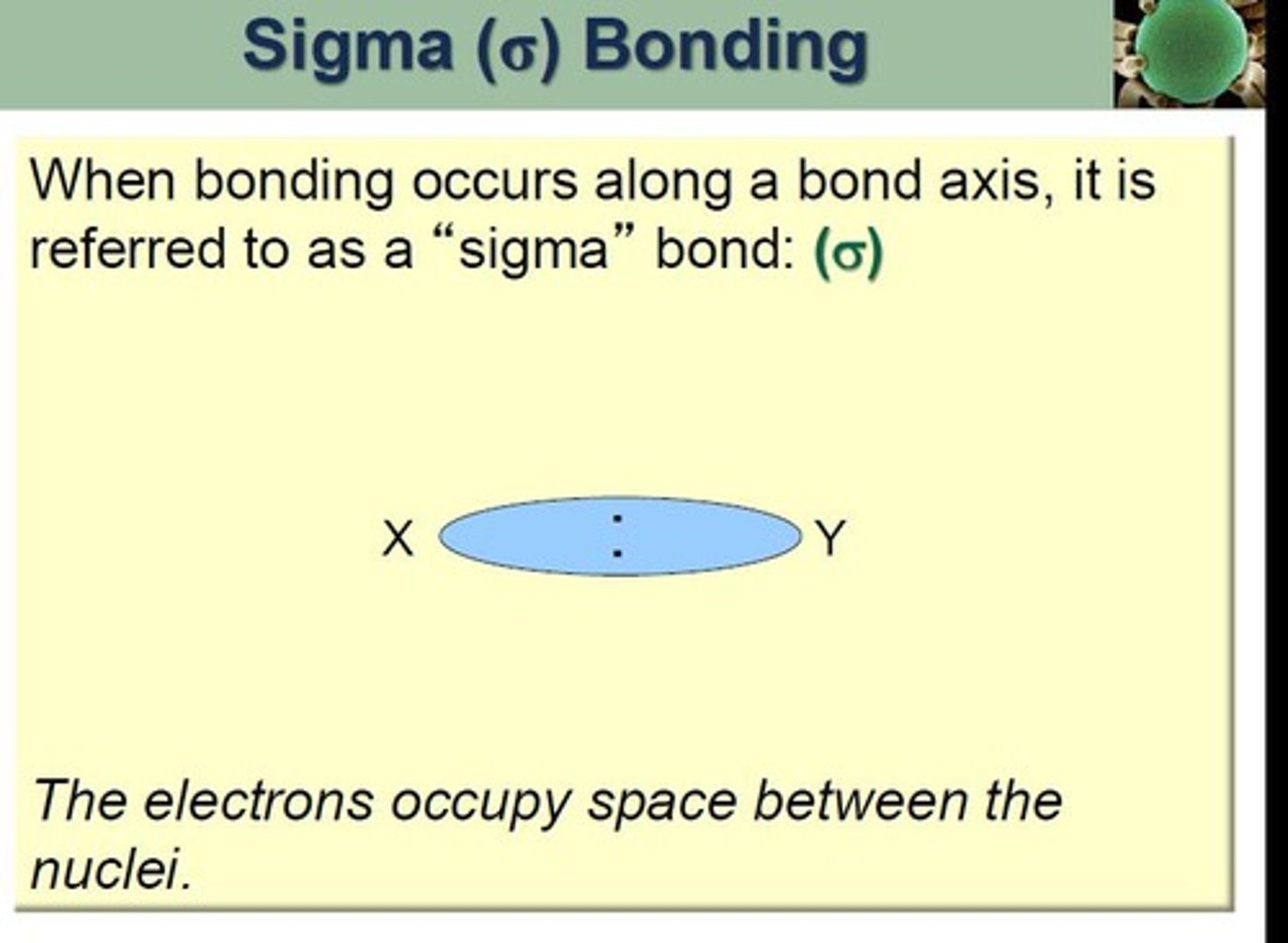
Sigma Bond (σ)
First bond formed by head-on orbital overlap.
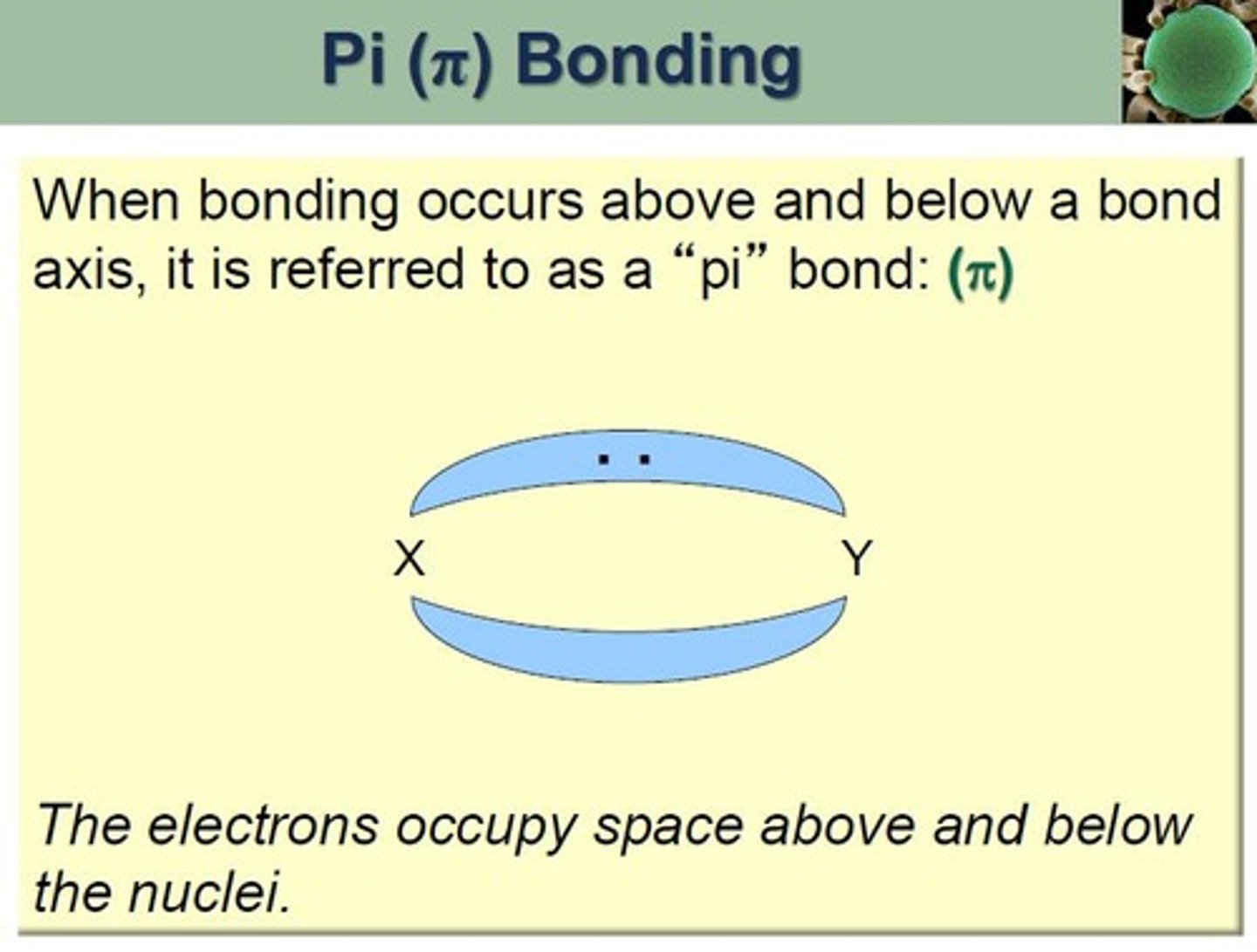
Pi Bond (π)
Bond formed by lateral overlap of p or d orbitals.
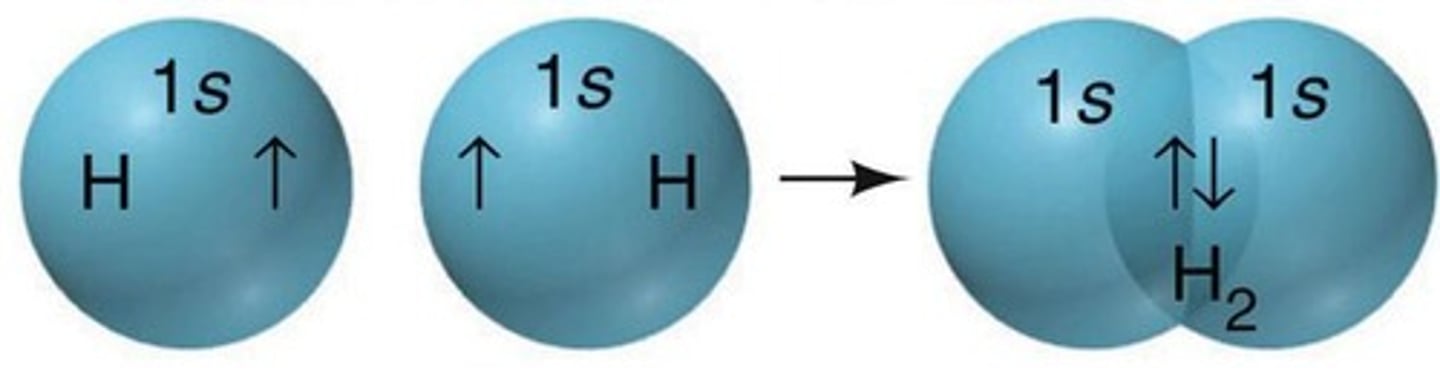
Orbital Overlap
Atomic orbitals overlap to form covalent bonds.
Bond Pair Electrons
Electrons occupying the overlapped region of orbitals.
Robert S. Mullikan
Developer of Molecular Orbital Theory.
End-to-End Overlap
Overlap type forming sigma bonds.
Sideways Overlap
Overlap type forming pi bonds.
Strength Order of Sigma Bonds
p-p > s-p > s-s in strength.
Hydrogen Atom Configuration
1s1 is the electronic configuration of hydrogen.
Fluorine Atom Configuration
2s2 2px 2py 2pz is fluorine's configuration.
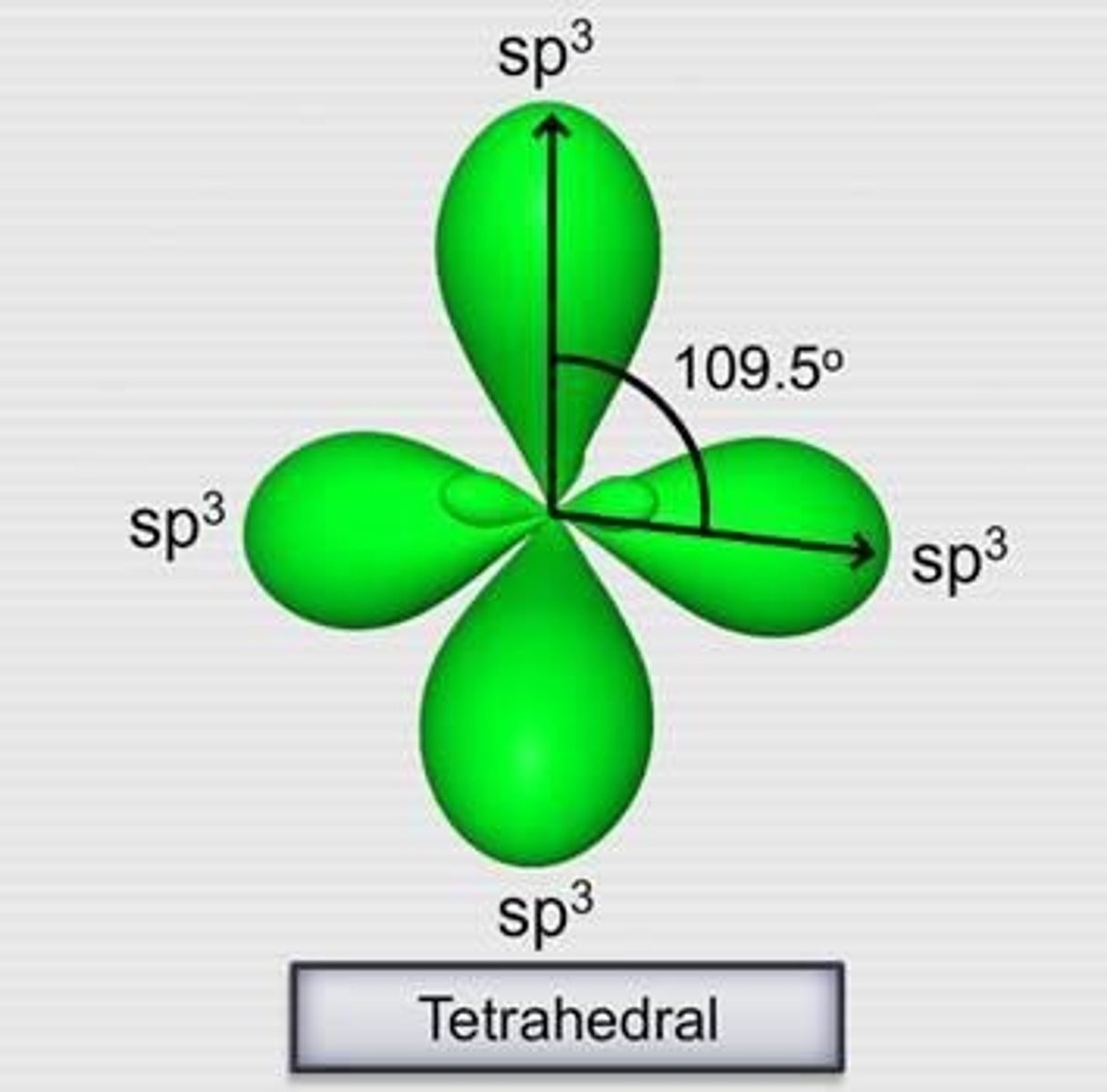
Tetrahedral Hybridization
sp3 hybridization with 109° bond angles.
Trigonal Hybridization
sp2 hybridization with 120° bond angles.
Digonal Hybridization
sp hybridization with 180° bond angles.
Hybrid Orbitals
New orbitals formed from hybridization of atomic orbitals.
Covalent Bond Formation
Result of orbital overlap between two atoms.
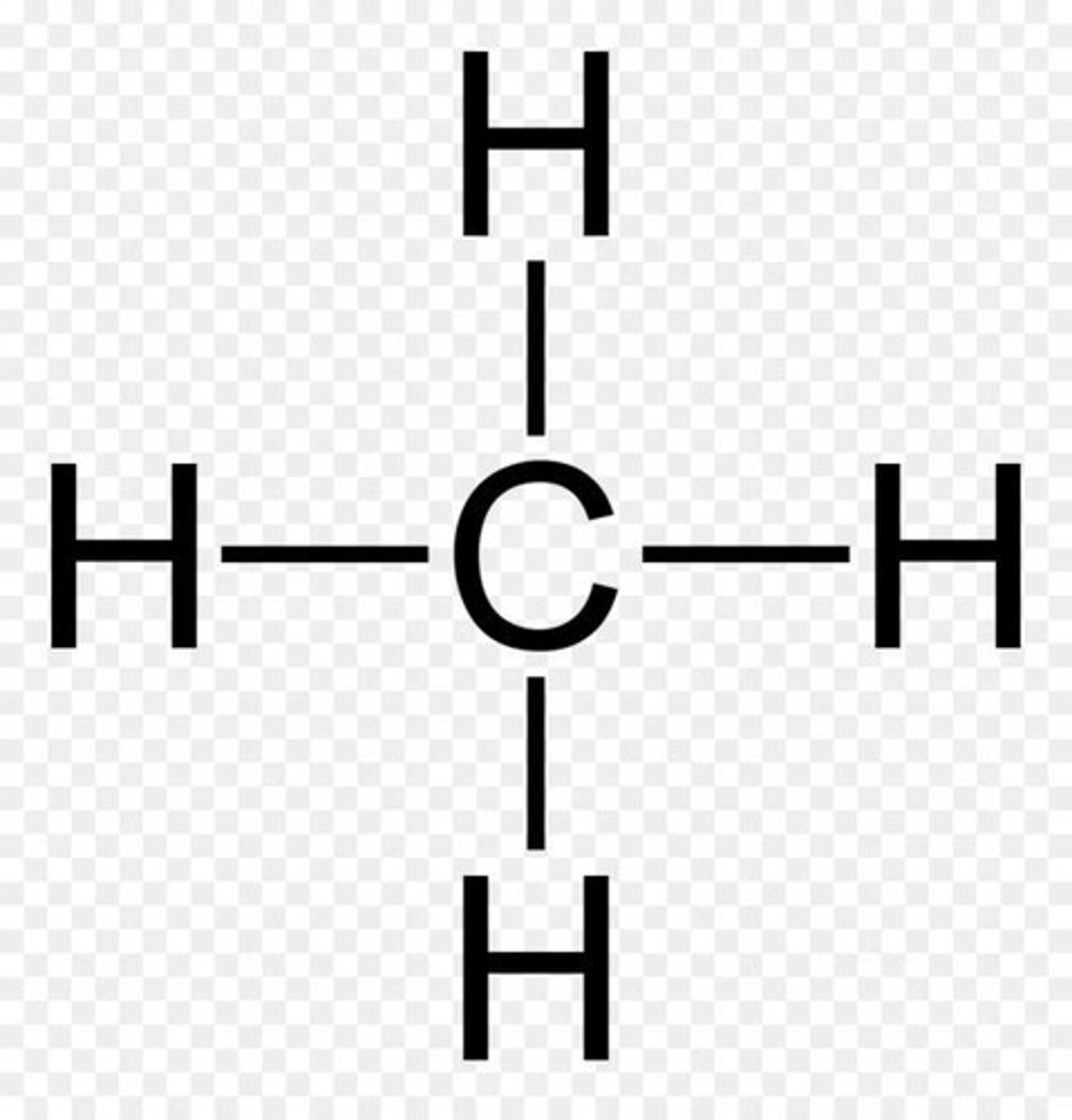
Methane (CH4) Structure
Carbon forms four equivalent sp3 hybrid bonds.
Ethylene (C2H4) Structure
Contains sp2 hybrid orbitals with 120° angles.
Acetylene (C2H2) Structure
Contains sp hybrid orbitals with 180° angles.
Electron Spin Pairing
Electrons in overlapping orbitals must have opposite spins.
Orbital Shapes
s = sphere, p = peanut, d = double peanut.
Bonding Electrons
Electrons that participate in bond formation.
Chemical Bonding
Attraction between atoms due to electron sharing.