2. Bonding of carbon and other atoms in organic molecules
0.0(0)
Card Sorting
1/7
Earn XP
Description and Tags
page 2
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
1
New cards
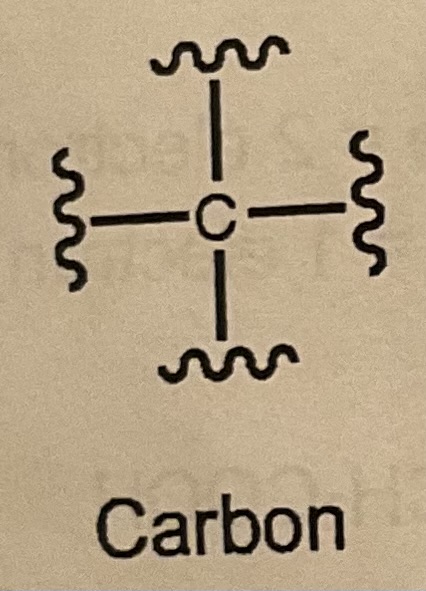
How many bonds/LP does Carbon want?
4 bonds and 0 LP
2
New cards
Bonds/LP for Oxygen?
2 bonds and 2 LP
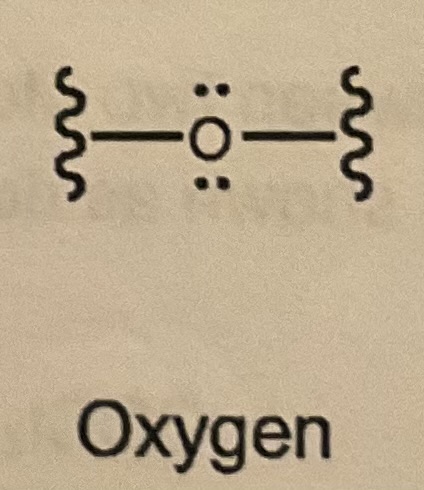
3
New cards
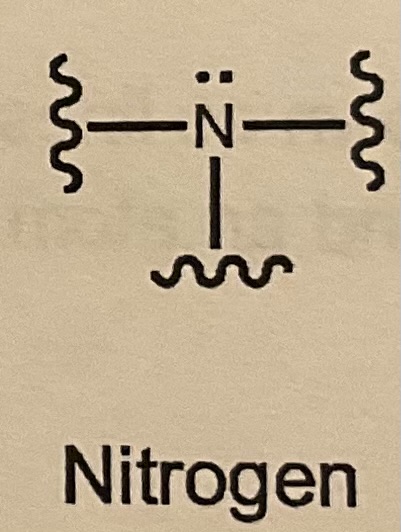
Bonds/LP for Nitrogen?
3 bonds and 1 LP
4
New cards
Bonds/LP for Halogens?
1 bond and 3 LP
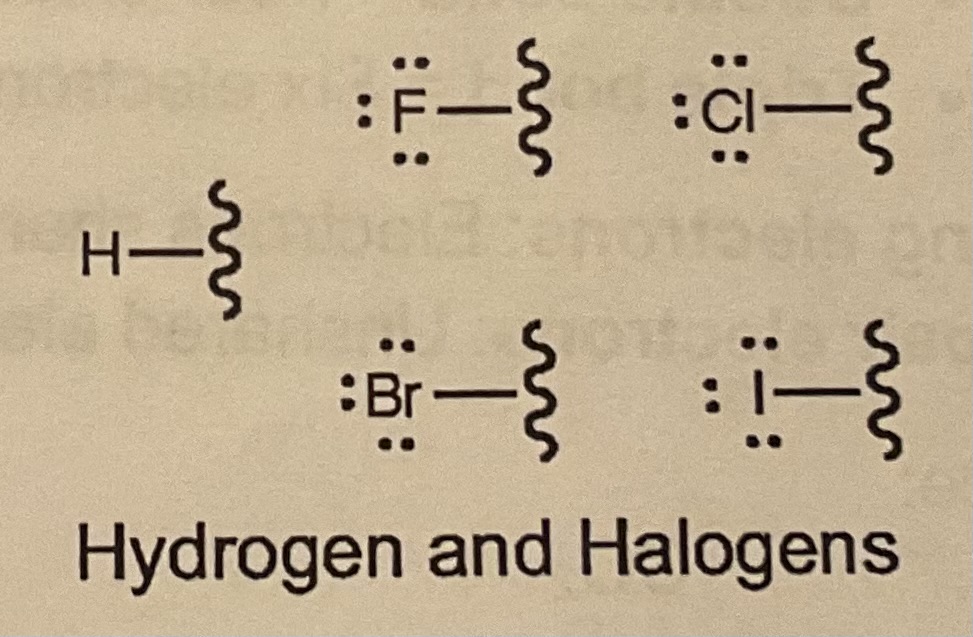
5
New cards
What 3 atoms can use their valence electrons to create double or triple bonds?
Carbon, Nitrogen, and Oxygen
6
New cards
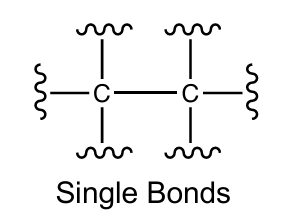
Single bond for carbon
1
7
New cards
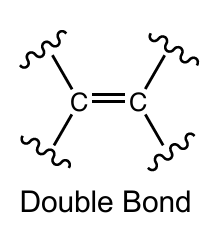
Double bond for carbon
2
8
New cards
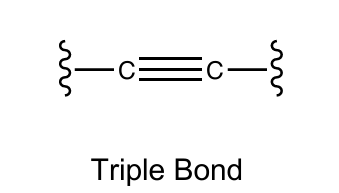
Triple bond for carbon
3