Module 2 ✅
1/157
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
158 Terms
What is the cell cycle process?
The cell spends most of its time in interphase, which consists of the G1, S and G2 phases
The next section is mitosis, when the nucleus divides
Then the cell undergoes cytokinesis, when the cell splits into two genetically identical daughter cells
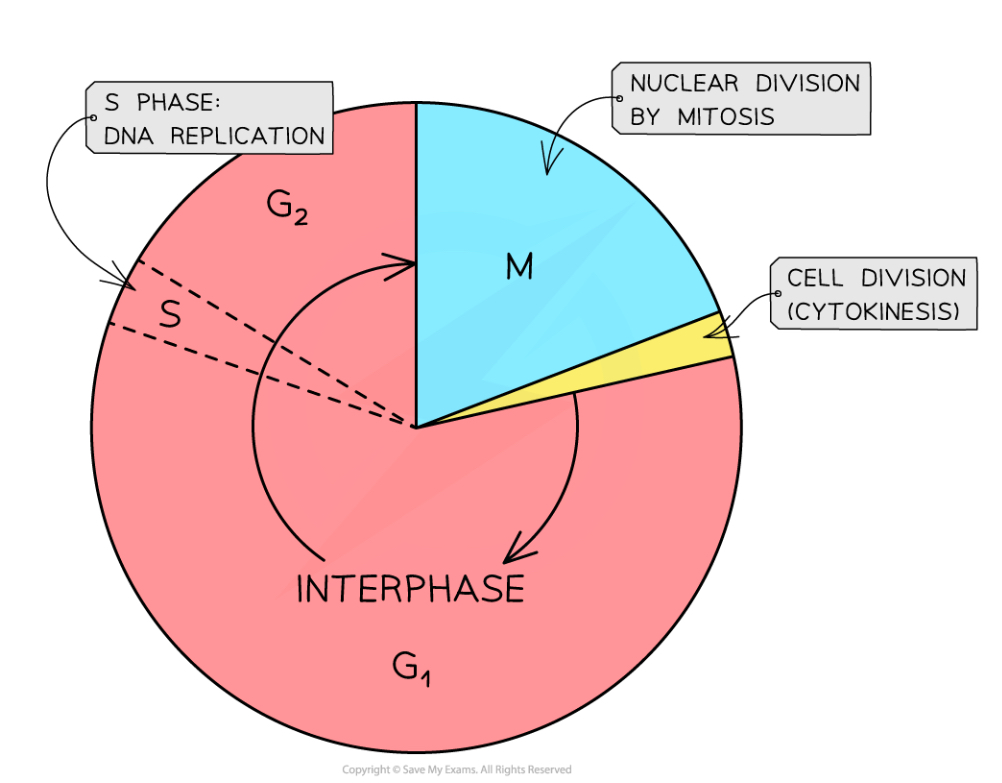
What happens in the cell during the G1 phase
G1 (1st growth phase of interphase)- the cell’s contents (except for chromosomes) duplicate, and proteins are synthesised
What happens in the cell during the S phase?
S (synthesis phase of interphase)- the DNA in the nucleus replicates, so each chromosome then is made up of two identical sister chromatids joined by a centromere
What happens in the G2 phase?
G2 (2nd growth phase of interphase)- the cell continues to grow and prepares for cell division (eg. produces tubulin protein for spindle fibres)
How is the cell cycle regulated?
Errors that occur in replicated DNA during the cell cycle are found and fixed at checkpoints in the process:
G1 checkpoint- after G1, the cell size, growth factors, nutrients and chromosomes are checked- if damage is detected in DNA then the cell enters a resting phase, G0, until it is repaired
G2 checkpoint- after G2, the cell size and replicated DNA are checked for errors- cells with incorrectly replicated DNA enter G0
Metaphase checkpoint- ensures the chromosomes are correctly attached to the spindle fibres during metaphase, so that anaphase occurs properly
When possible enzymes will repair the error but sometimes the cell destroys itself to prevent passing on harmful mutations
A cell can also enter G0 when it has become too differentiated to divide further
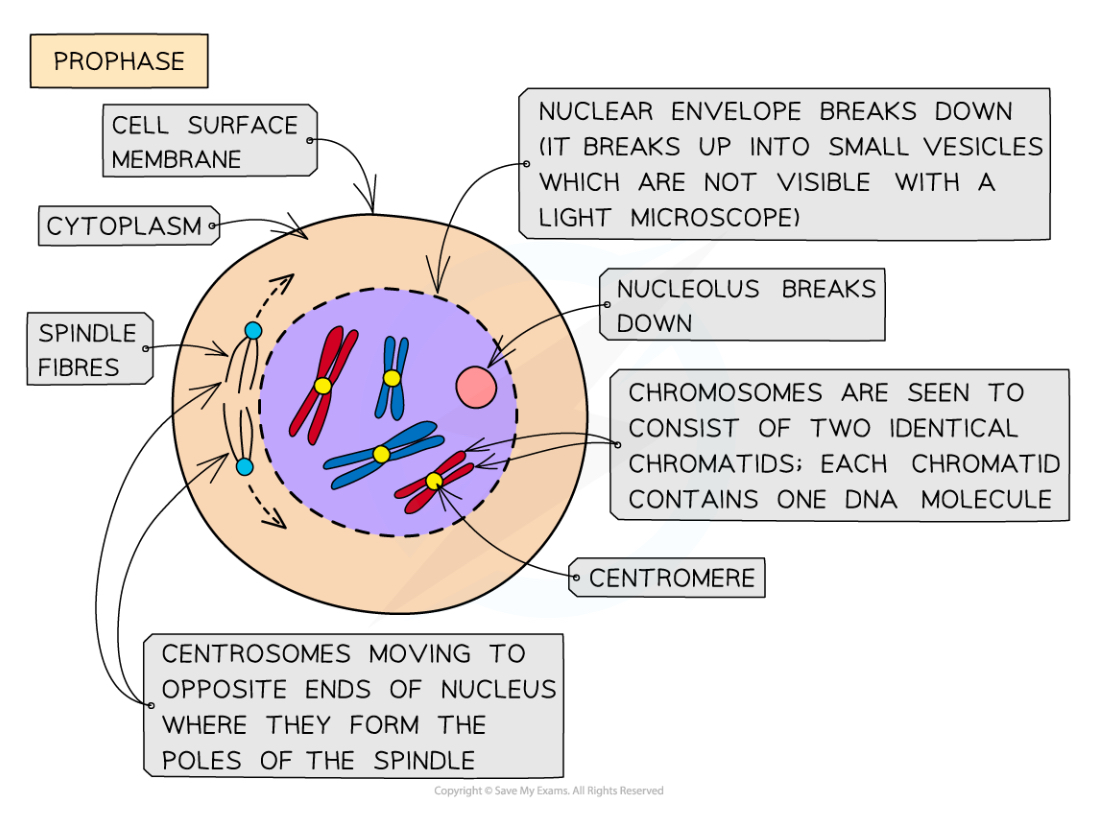
What happens in prophase during mitosis?
Chromosomes condense (can be seen as two sister chromatids that are joined together at the centromere when stained)
Two centrioles move towards opposite poles of the cell
Spindle fibres (microtubules) begin to form at the centrioles
The nuclear membrane breaks down
The nucleolus disappears
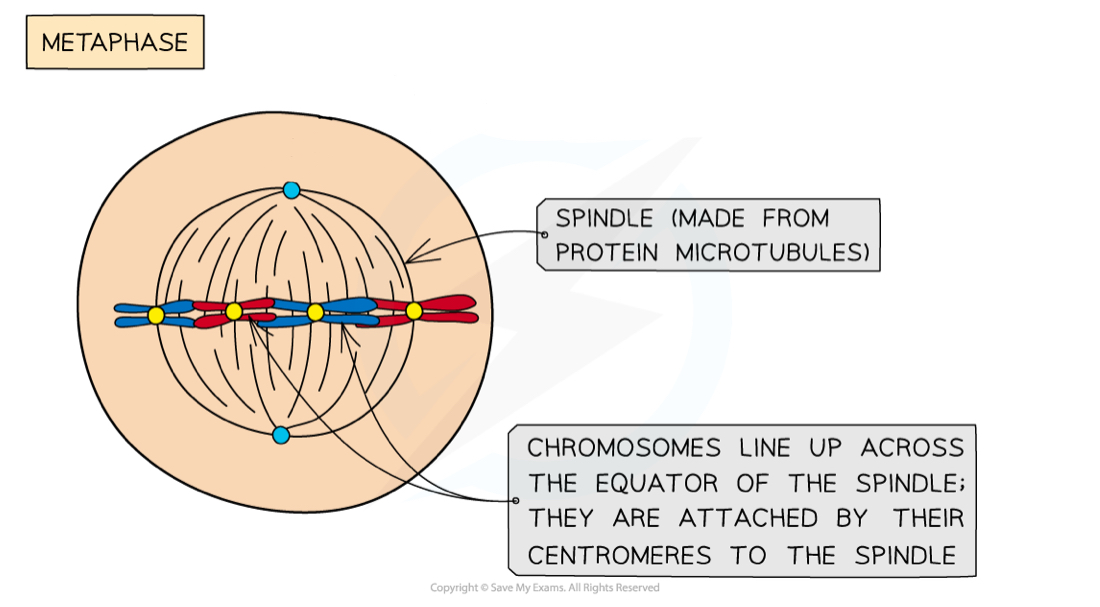
What happens in metaphase during mitosis?
Spindle fibres attach to the centromere of each chromosome
This attaches the chromosomes to each centriole, and they line up along the equator
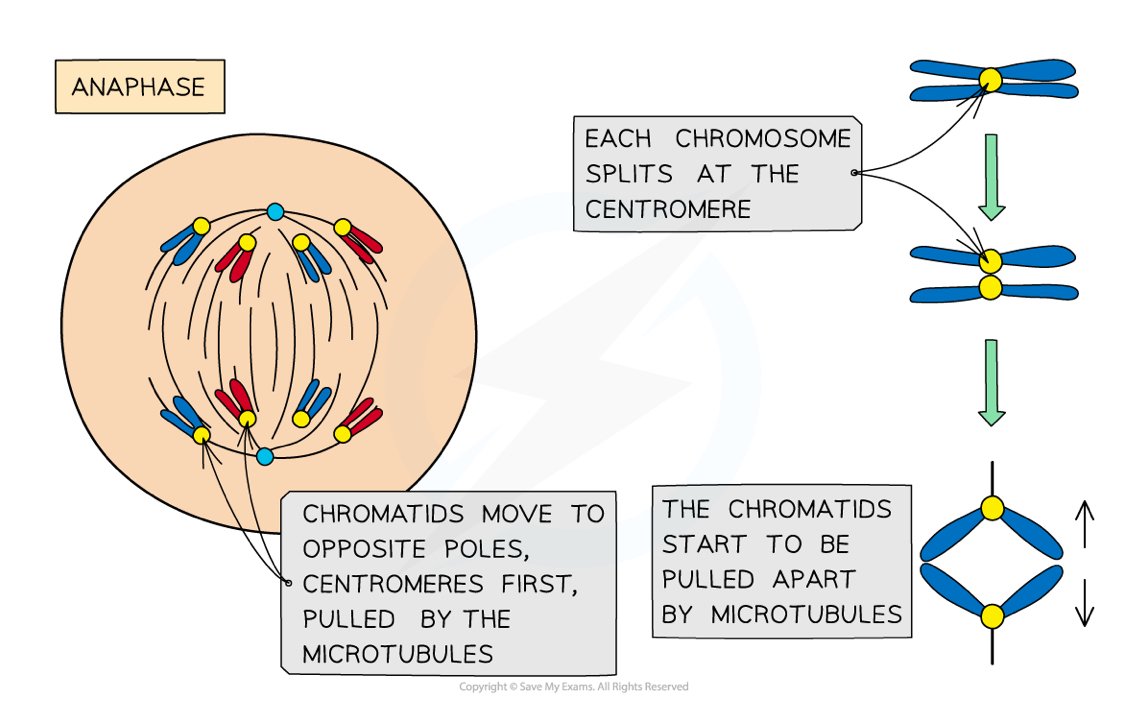
What happens in anaphase during mitosis?
Spindle fibres contract, dividing each centromere in half
The chromosomes split into their two daughter chromatids
The chromatids move towards the poles, so that both poles have one half of each original chromosome
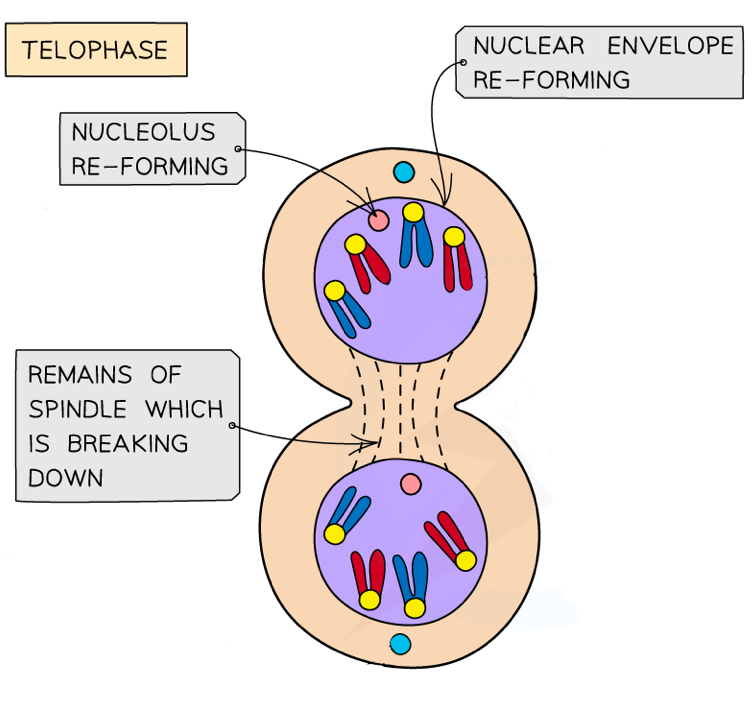
What happens in telophase during mitosis?
Chromosomes (now made up of only one chromatid again) reach each pole and decondense
Nuclear membranes reform around the groups of chromosomes
Nucleoli reform
Spindle fibres break down
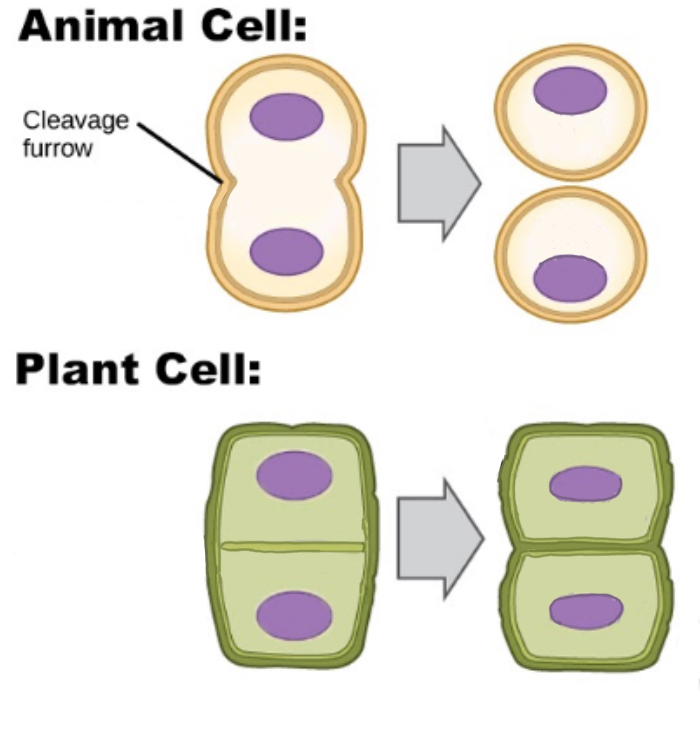
What happens in cytokinesis during mitosis?
In animal cells, the cell membrane pinches and cleaves, dividing the cell into two
In plant cells, the cell membrane and cell wall form so that the two new cells still remain connected
Why is mitosis important?
Growth- mitosis produces two daughter cells which are genetically identical to the parent cell, allowing organisms to grow
Tissue repair- as cells are constantly dying they are replaced by identical cells
Asexual reproduction- plants, fungi and some animal species can reproduce without a second parent using mitosis
What happens in prophase 1 in meiosis?
Chromosomes condense (can be seen as two sister chromatids that are joined together at the centromere when stained)
Two centrioles move towards opposite poles of the cell
Spindle fibres (microtubules) begin to form at the centrioles
The nuclear membrane breaks down
The nucleolus disappears
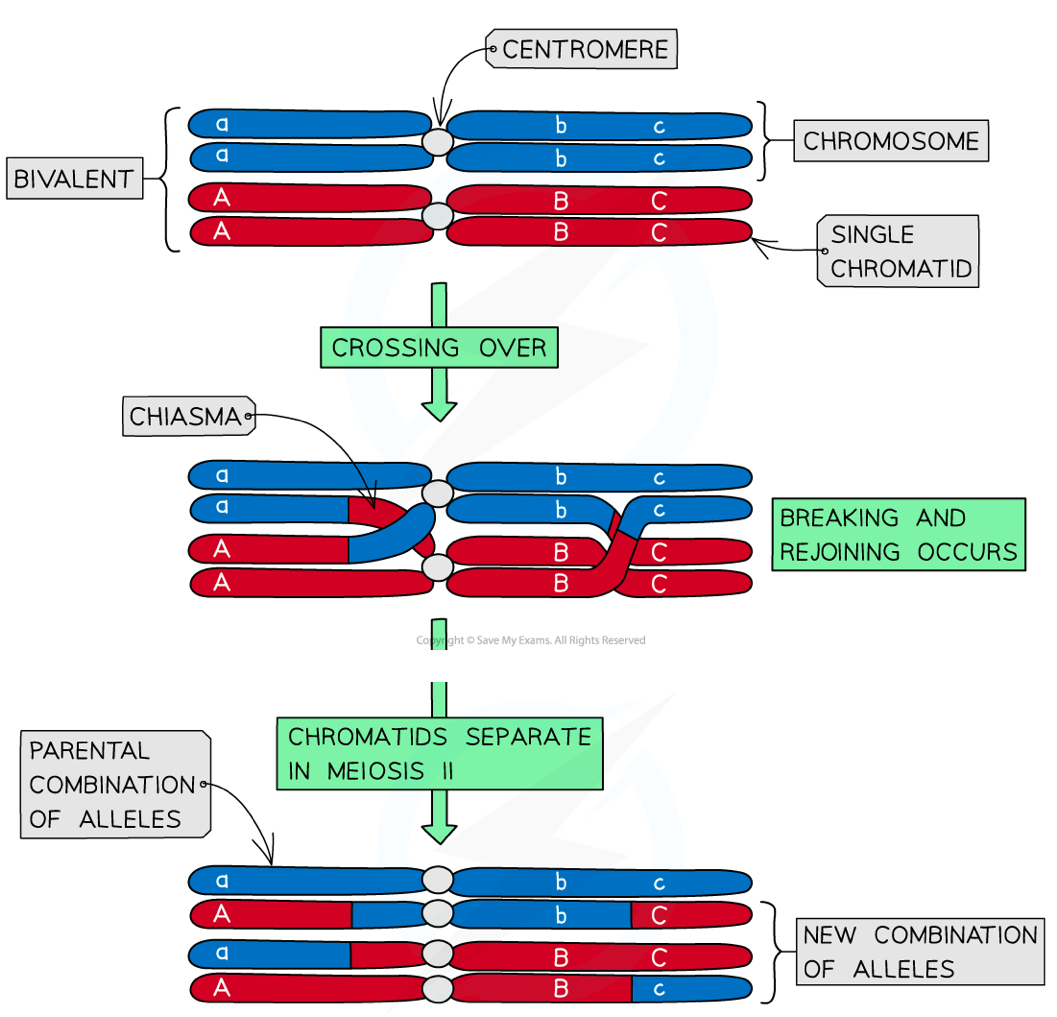
The chromosomes are arranged side by side in homologous pairs (forming bivalents)
As the homologous chromosomes are very close together the crossing over of certain genes at chiasmata between chromosomes may occur- this produces genetic variation
Prophase 2 is the same as prophase in mitosis
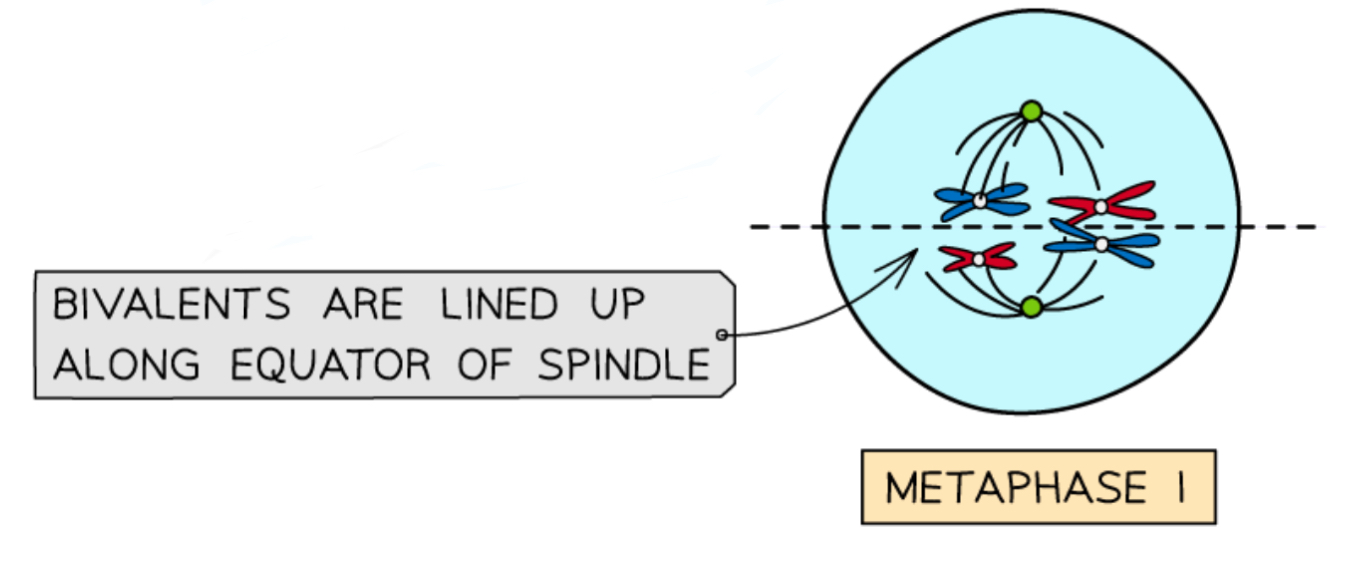
What happens in metaphase 1 in meiosis?
The bivalents (pairs of homologous chromosomes) line up at the equator of the cell
Spindle fibres attach to the centromere of each chromosome
The maternal and paternal chromosome in each bivalent line up on either side randomly in an independent assortment- this produces genetic variation
Metaphase 2 is the same as metaphase in mitosis
There is also independent assortment in metaphase 2, as the chromosomes line up randomly (can have either sister chromatid facing each pole)
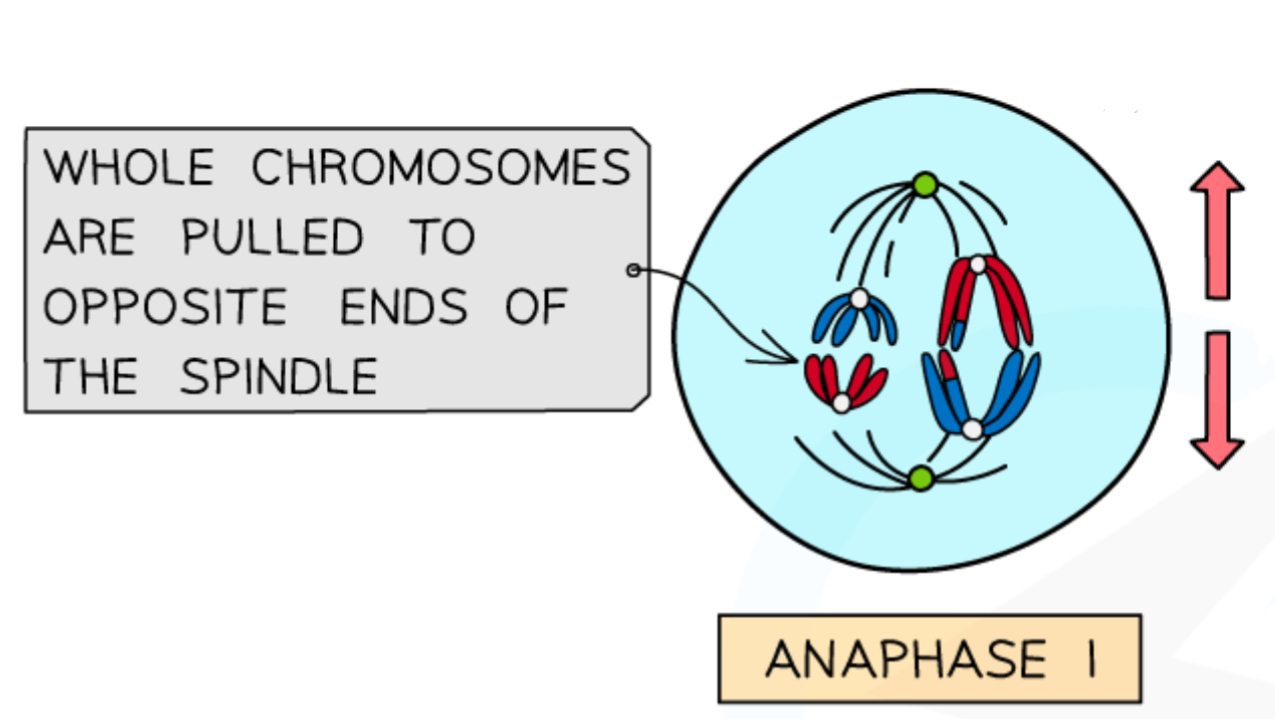
What happens in anaphase 1 in meiosis?
Spindle fibres contract, pulling whole chromosomes to each pole
The bivalents are split up, so both poles have a version of each chromosome (which still have two chromatids joined by a centromere)
Meiosis 1 is called reduction division because the chromosome number of resulting cells is halved, due to anaphase 1
Anaphase 2 is the same as anaphase in mitosis
What happens in telophase 1 in meiosis?
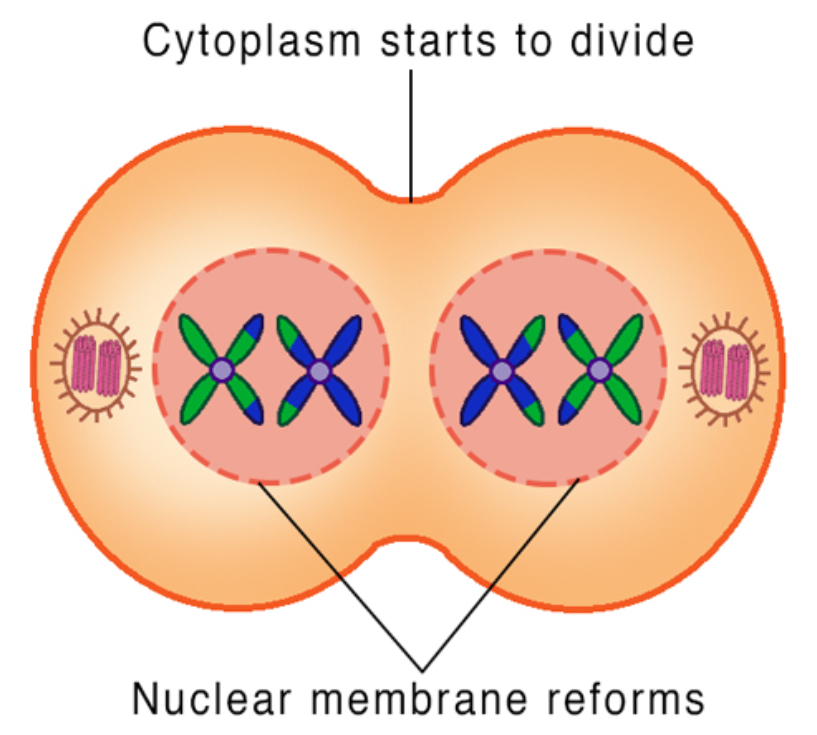
Chromosomes (still made up of two chromatids joined by a centromere) reach each pole
Nuclear membranes reform around the groups of chromosomes
Nucleoli reform
Spindle fibres break down
Telophase 2 is the same as telophase in mitosis
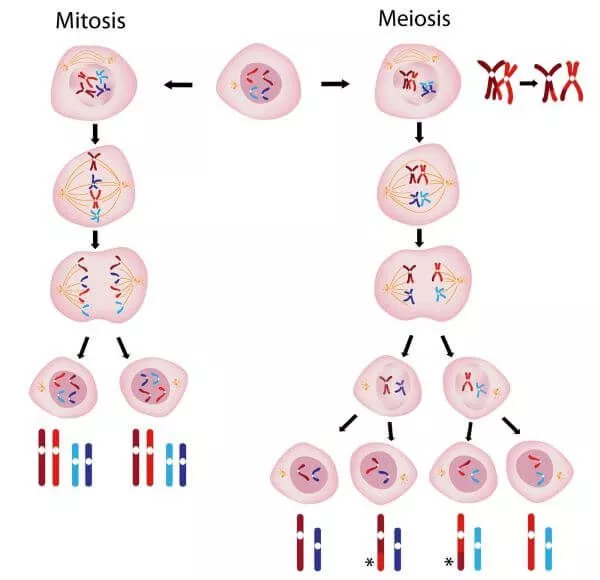
Mitosis vs meiosis process
There is an interphase stage before meiosis 1 but not before meiosis 2
Meiosis 1 produces haploid cells, but with chromosomes that have two chromatids
Meiosis 2 produces haploid cells where the chromosomes have one chromatid
Why is meiosis important?
Sexual reproduction:
Meiosis produces cells with half the number of chromosomes as in a normal cell, which means it is important for producing gametes
Gametes, sperm and egg cells, need to be haploid so that they produce a diploid cell when they fuse
Produces genetic variation:
Crossing over allows the exchange of genes between maternal and paternal chromosomes in a bivalent
Independent assortment means there are often millions of combinations of alleles that will be passed on as the chromosomes in a bivalent arrange randomly
How are erythrocytes specialised?
Red blood cells transport oxygen and carbon dioxide, so they:
Contain haemoglobin that binds to oxygen
Have no nucleus
Are biconcave and flattened so they have a higher s.a and a short diffusion pathway
Are small to fit through capillaries
Contain carbonic anhydrase to transport CO2
How are neutrophils specialised?
White blood cells protect the body from pathogens, so they:
Have lysosomes that contain digestive enzymes
Have multi-lobed nuclei so they can fit through small gaps
Specific receptors on surface
Flexible shape
How are sperm cells specialised?
Sperm cells fertilise egg cells, so they:
Have a flagellum for movement
Have lots of mitochondria to release energy
Are haploid so they produce a diploid cell after fertilisation
Have acrosome enzymes at the head to penetrate the egg cell
How is squamous epithelium tissue specialised?
Squamous epithelium lines the lungs, so it:
Is one cell thick to allow fast diffusion of oxygen into the blood
How is ciliated epithelium tissue specialised?
Ciliated epithelium lines the trachea and fallopian tubes, so it:
Has cilia that beat in rhythm and sweep particles
Move bacteria, mucus, etc in trachea to protect lungs
Move egg cells in the fallopian tubes
Contain goblet cells that release mucus to trap particles in trachea
How is cartilage tissue specialised?
Cartilage is a connective tissue, so it:
Contains elastin + collagen
Is firm but flexible
Composed of chondrocyte cells in a matrix
How is muscle tissue specialised?
Muscle is used for movement, so it:
Contains contractile proteins
Can contract + lengthen to move bones
Contain many mitochondria for energy
Can be skeletal, cardiac or smooth
How are palisade cells specialised?
Palisade cells are the main site of photosynthesis, so they:
Contain many chloroplasts
Are closely packed
Have thin cell walls for carbon dioxide diffusion
Chloroplasts can move towards light
How are root hair cells specialised?
Root hair cells absorb water and mineral ions, so they:
Have a high surface area to increase diffusion
Have many mitochondria for active transport
How are guard cells specialised?
Pairs of guard cells form stomata, so they:
Are very subject to turgidity changes so they can close + open stomata
Thick inner cell wall
Control gas exchange and water loss
How is epidermis tissue specialised?
Epidermis covers the surface of plants, so it:
Has a thick, waxy cuticle to reduce water loss
Contain stomata
Clear + thin, so light can still get through for photosynthesis
How is xylem tissue specialised?
Xylem transports water + mineral ions, so it:
Is made of dead cells hollowed into tubes
Is strengthened by lignin to withstand the pressure of the transpiration stream
Only allows movement from roots to shoots
How is phloem tissue specialised?
Phloem transports sucrose by translocation, so it:
Is made up of living sieve and companion cells
Has pores between cells to allow sucrose to move
Has little cytoplasm to allow for more substances to be transported
Allows movement in both directions
Has many mitochondria in companion cells for active transport
Has large vacuole in companion cells for storage
What is totipotency?
Totipotent stem cells can differentiate into any cell type, and can produce a whole organism
They are found in zygotes (up to 16 cells)
What is pluripotency?
Pluripotent stem cells can differentiate into any cell type, but can’t produce a whole organism
Found in early embryos and the meristem of plants- the apical meristem (cambium) at the tips of roots and shoots differentiates into xylem and phloem tissues
What is multipotency?
Multipotent stem cells can only differentiate into a few cells within a tissue
Adult stem cells found in bone marrow can only differentiate into different blood cells (erythrocytes and neutrophils)- they replace these constantly
How can stem cells be used in medicine?
Replacing:
Damaged heart muscle tissue to treat heart disease
Insulin-producing cells in the pancreas to treat type 1 diabetes
Dead dopamine-providing cells in the brain to treat Parkinson’s
Dead nerve cells in the brain to treat Alzheimer’s
Damaged retinal cells in the eye to treat macular degeneration
Blood cells to treat blood diseases like leukaemia
Damaged cells in the spinal cord to treat paralysis
Producing cell cultures for drug trials
Studying developmental biology
differences between RNA and DNA nucleotides?
RNA is a ribose sugar rather than deoxyribose
the thymine base is replaced with uracil [still pyrimidine]
![<ul><li><p>RNA is a ribose sugar rather than deoxyribose </p></li><li><p>the thymine base is replaced with uracil [still pyrimidine]</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/027abb49-9a95-4d35-9b21-fbc2392d27bc.jpeg)
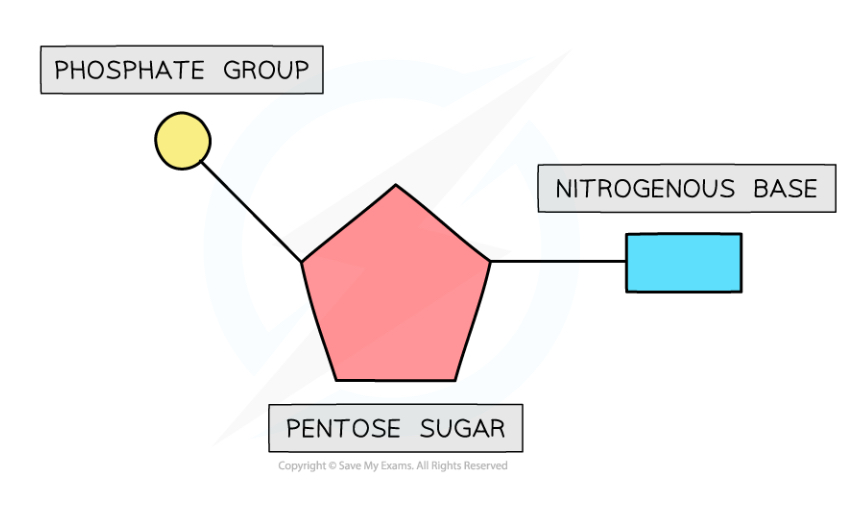
Structure of a nucleotide
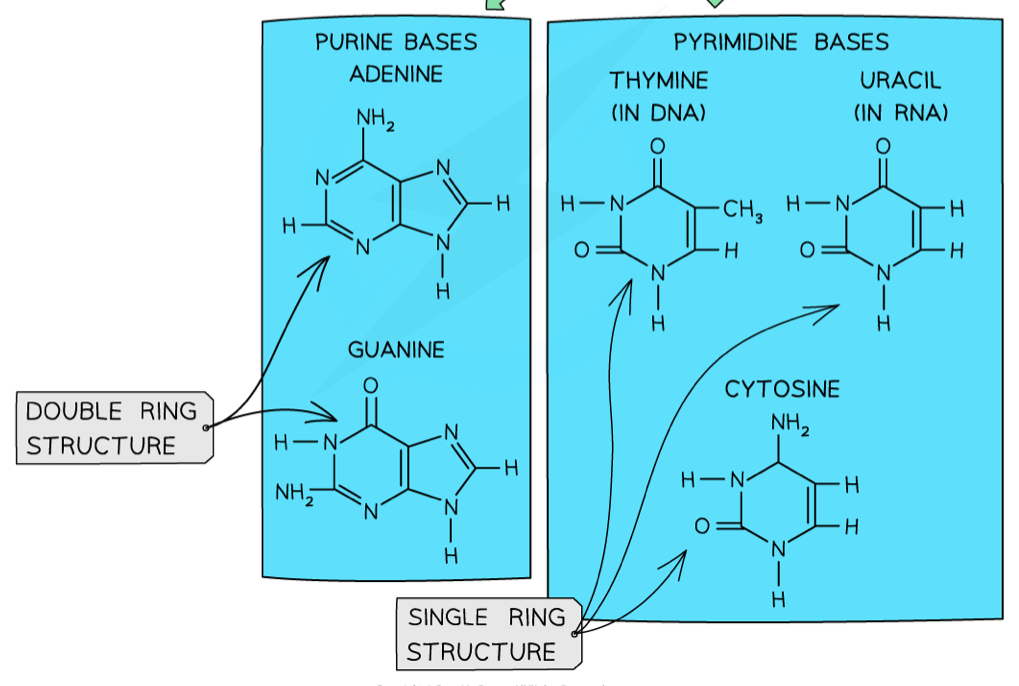
What are the purine and pyrimidine bases?
Purine: A and G (double ring)
Pyrimidine: T, U and C (single ring)
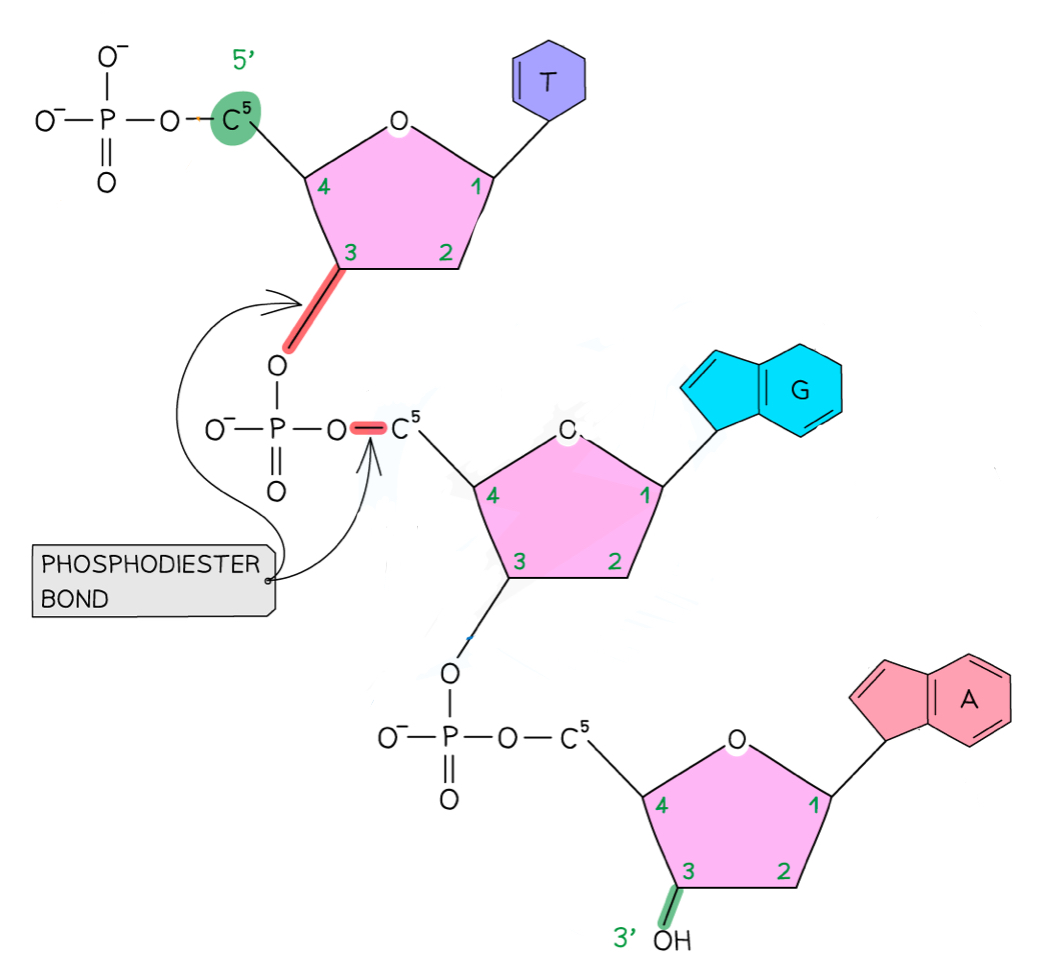
How is the sugar-phosphate backbone formed?
Condensation reactions forming phosphodiester bonds between the phosphate group from one nucleotide and the pentose sugar of another
The 5-carbon of one sugar is linked to its phosphate group, which is then linked up to the 3-carbon of the sugar from the next nucleotide
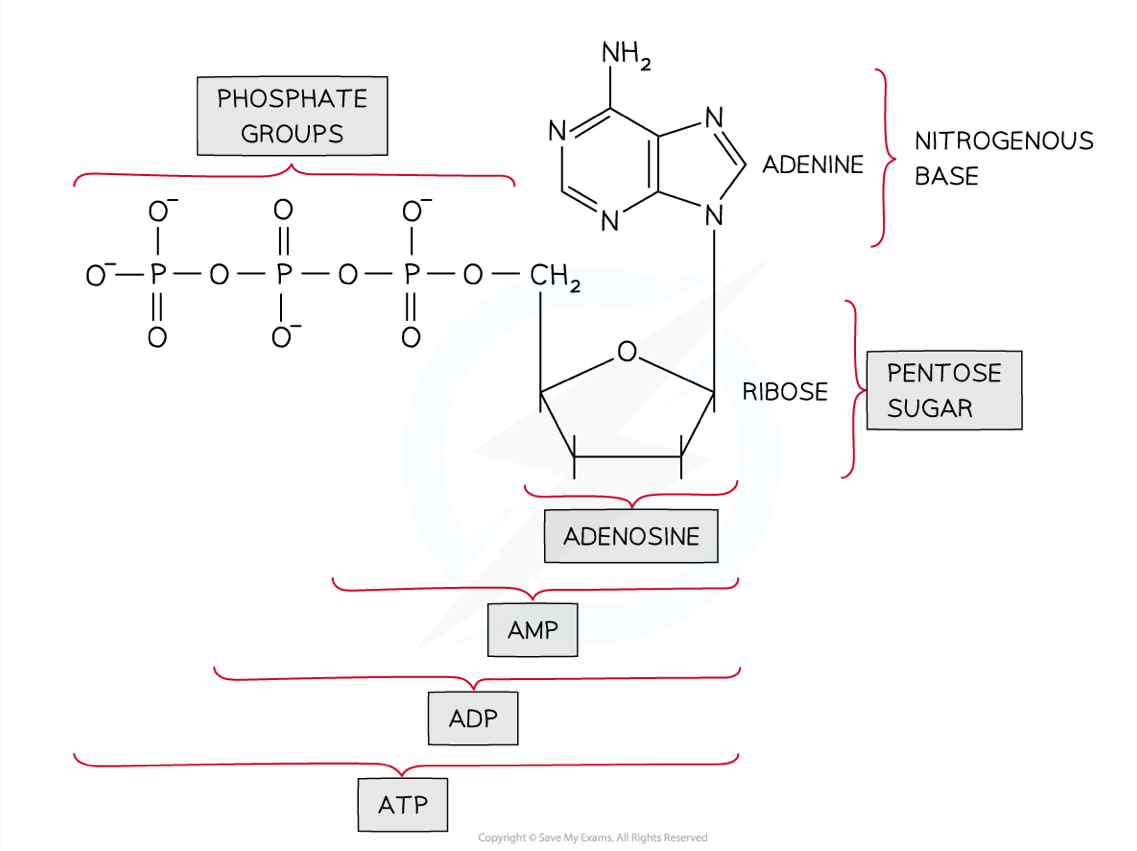
What are the structures of ATP and ADP?
ATP and ADP (adenine triphosphate and adenine diphosphate) are phosphorylated nucleotides
They are made up of adenine bonded to a ribose sugar and three (ATP) or two (ADP) phosphate groups
How is ATP used?
synthesis - of large molecules
transport - pumping molecules across cell membranes
movement - protein fibres in muscle cells
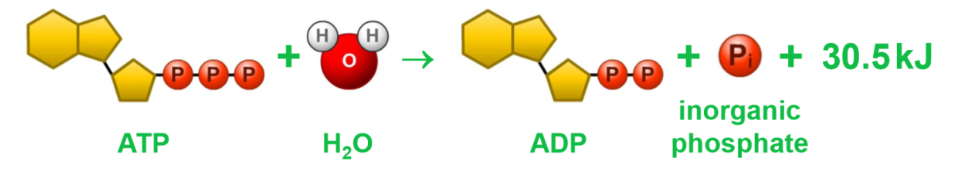
In cells, one of the phosphate groups in ATP is removed by a hydrolysis reaction, to produce ADP and release energy
This energy is used in cellular processes, mainly synthesis, transport and movement
ATP is resynthesized from ADP in concentration reactions
ATP is relatively unstable, but it is a good immediate energy source
What properties of ATP make it a good immediate energy source?
Small- easy to transport
Water soluble- can be used in aqueous environments
Phosphate group bond has energy- enough to be used in reactions, but not so much that energy is wasted
Easily regenerated- the ATP → ADP hydrolysis reaction is easily reversed in a condensation reaction
What is the structure of DNA?
Two polynucleotide strands connected by hydrogen bonds between bases, which are antiparallel (run in opposite directions- one is 3’ to 5’ and the other is 5’ to 3’),
twisted into a double helix
bases bind - complimentary base pairing [tea for 2]
![<ul><li><p><strong>Two polynucleotide strands</strong> connected by hydrogen bonds between bases, which are <strong>antiparallel </strong>(run in opposite directions- one is 3’ to 5’ and the other is 5’ to 3’),</p></li><li><p>twisted into a double helix</p></li><li><p>bases bind - <strong>complimentary base pairing [</strong>tea for 2]</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/9f06fdb6-1b70-4f9e-b8a9-b1b814af6428.jpeg)
How can we purify DNA and what does each step do?
Add detergent, - breaks down cell membrane releasing cell contents
Add salt- breaks hydrogen bonds between DNA and water
Add protease - protease enzymes break down the histones that hold DNA together, and the salt neutralises the charge of the phosphate backbone
Heat in a water bath- more KE provides a higher rate of reaction, and the heat denatures enzymes in the cells that would break down the DNA further
Add ice cold ethanol- DNA is not soluble in alcohol, so adding ice cold ethanol allows the DNA to precipitate on top of the solution. You can then take out this precipitate with a glass rod
What do we call the process by which DNA is copied and why?
Semi-conservative replication, because in each of the two DNA molecules produced, one of the polynucleotide strands comes from the original DNA molecule being copied, and the other strand is new
What is the process of semi-conservative replication?
The DNA helix untwists
DNA helicase breaks the hydrogen bonds between the bases, separating the two strands
Complementary free nucleotides join up with the exposed bases on the strand, by hydrogen bonds
DNA polymerase catalyses the condensation reactions of phosphodiester bonds between the bases to form a new strand
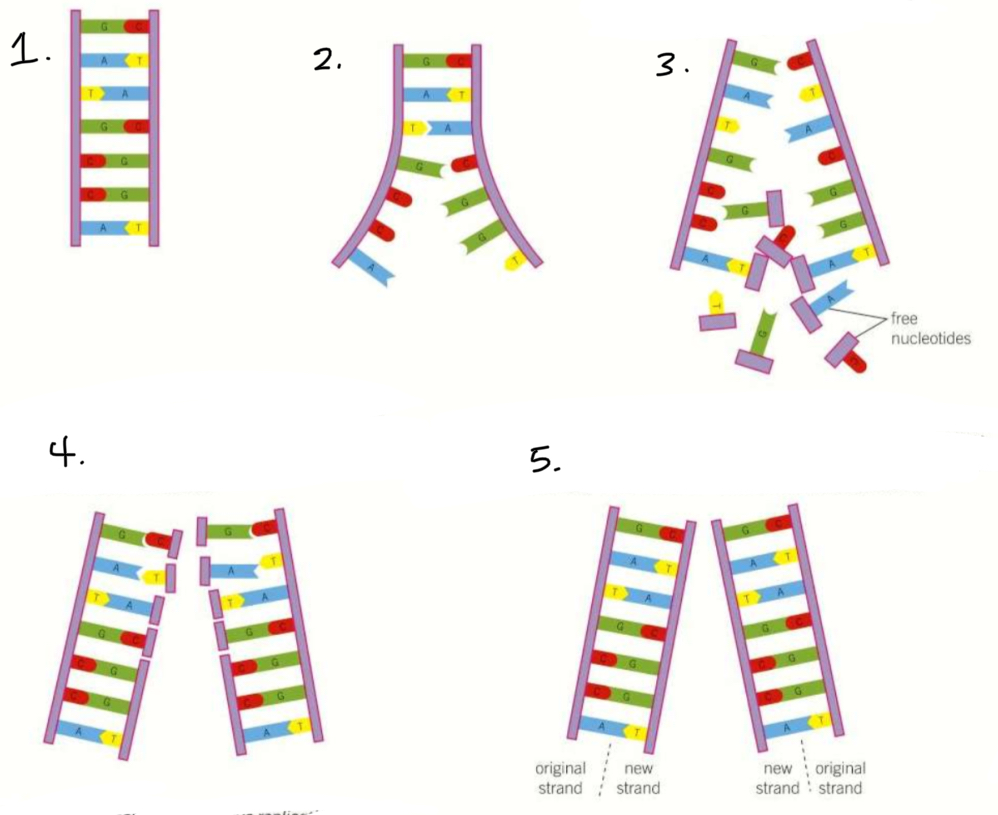
Why does the DNA replication process have to be very accurate?
The daughter DNA molecules must be an exact copy of the parent DNA molecule, avoiding mutations where:
Bases are inserted into the complementary strand in the wrong order
An extra base is inserted by accident
A base is left out
Mutations can have a big impact on the structures produced from the DNA, particularly for enzymes (proteins), which need a specific shape
What is a gene?
A sequence of bases in a DNA molecule that codes for a protein
a gene determines the sequence of amino acids in a polypeptide (the primary structure of a protein)
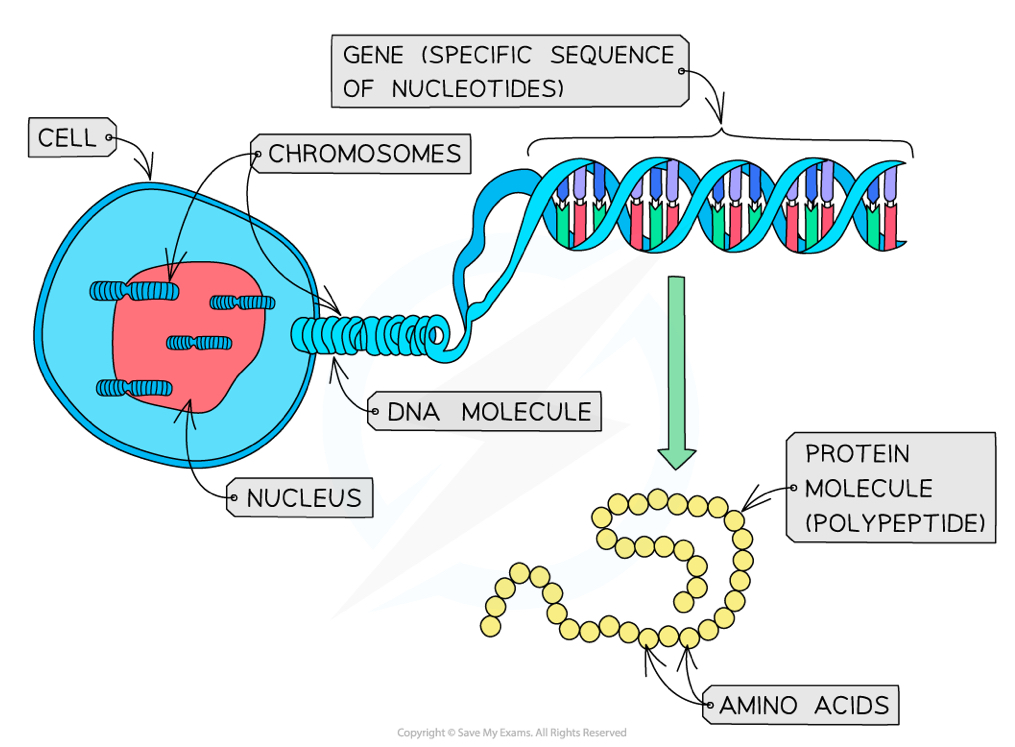
what are the 4 properties of the genetic code?
triplet code
universal
degenerate
non-overlapping
What does a triplet code mean?
Each sequence of three bases in a gene codes for one amino acid (of twenty)- in translation this is called a codon
How is the genetic code non-overlapping?
Genes are read in discreet triplets (codons), they do not overlap
How is the genetic code degenerate?
Multiple triplets of bases (codons) can code for the same amino acid
How is the genetic code universal?
The triplets of bases (codons) code for the same amino acids in all organisms
What is the process of transcription?
In the nucleus, part of a DNA molecule unwinds
DNA helicase breaks the hydrogen bonds between bases to split it into two strands
RNA polymerase moves along the template (sense) DNA strand, pairing the exposed bases with complementary ones, but replacing T with U
The nucleotides join up by phosphodiester bonds to create a strand of mRNA
The mRNA leaves the nucleus to a ribosome
The DNA rejoins into a double helix
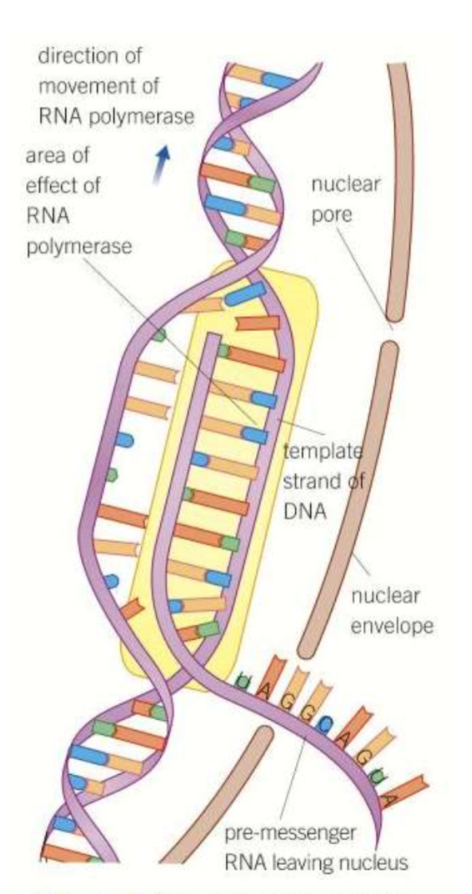
What is the process of translation?
The mRNA strand joins with a ribosome (made of rRNA)
At the first codon of mRNA, a tRNA molecule binds, which has a complementary anticodon
This repeats for each codon
Each tRNA molecule carries a specific amino acid, which join together by peptide bonds to form a polypeptide chain
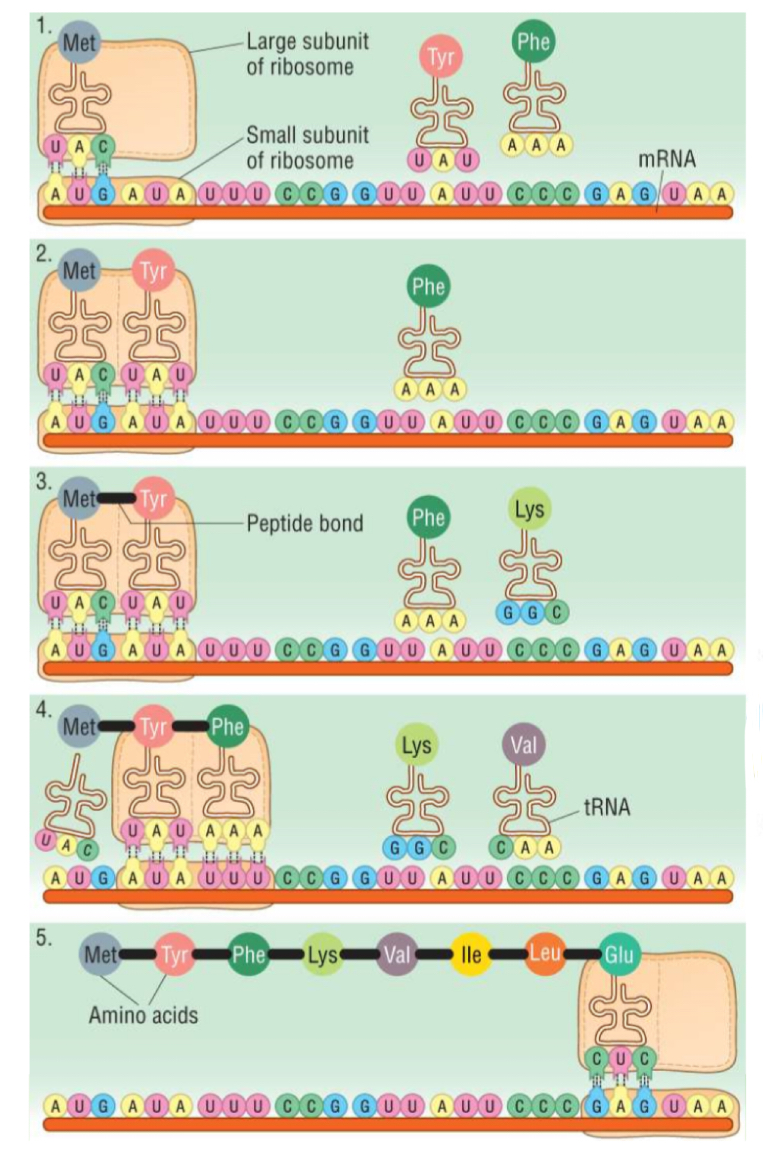
Why is mRNA used in protein synthesis?
DNA is too big to fit through nuclear pores, but mRNA is small enough to be able to
the role of enzymes in catalysing reactions that affect metabolism at a cellular and whole organism level
anabolic and catabolic reaction pathways
digestion of starch:
starch polymers are partially broken down into maltose by amylase [produced at salivary glands and pancreas
maltose is then broken down into glucose by maltase [in the small intestine]
What are intracellular enzymes? Include an example
Enzymes that are produces and function inside cells
Catalase is an intracellular enzyme that converts toxic hydrogen peroxide into water and oxygen
What are extracellular enzymes? Include two examples
Enzymes that are secreted from cells and function outside them
Trypsin is an extracellular enzyme which breaks down proteins into shorter peptide chains and amino acids
Starch is too big of a molecule to enter cells, so the enzyme amylase is involved in its digestion by hydrolysis into simple sugars, excreted from the salivary glands and the pancreas
Compare the two models for enzyme action
Lock and key hypothesis:
Enzymes have a highly specific 3D tertiary structure as they are globular proteins
So each enzymes active site is complementary to its substrate; they fit together exactly to form enzyme-substrate complexes
the substrate/substrates then react the products: enzyme product complex are released
held in such a way that atom groups are close enough to react
R groups within the active site interact with the substrate put a strain on the bonds
Induced fit hypothesis:
The active site and the substrate are not exactly complementary in shape
The enzyme and its active site can undergo slight conformational changes (changes in shape) to form enzyme-substrate complexes
initial interaction between enzyme and substrate is relatively weak but the interactions rapidly induce changes in the enzymes tertiary structure that strengthen binding putting a strain ont he substrate molecule
lowering the activation energy for the reaction
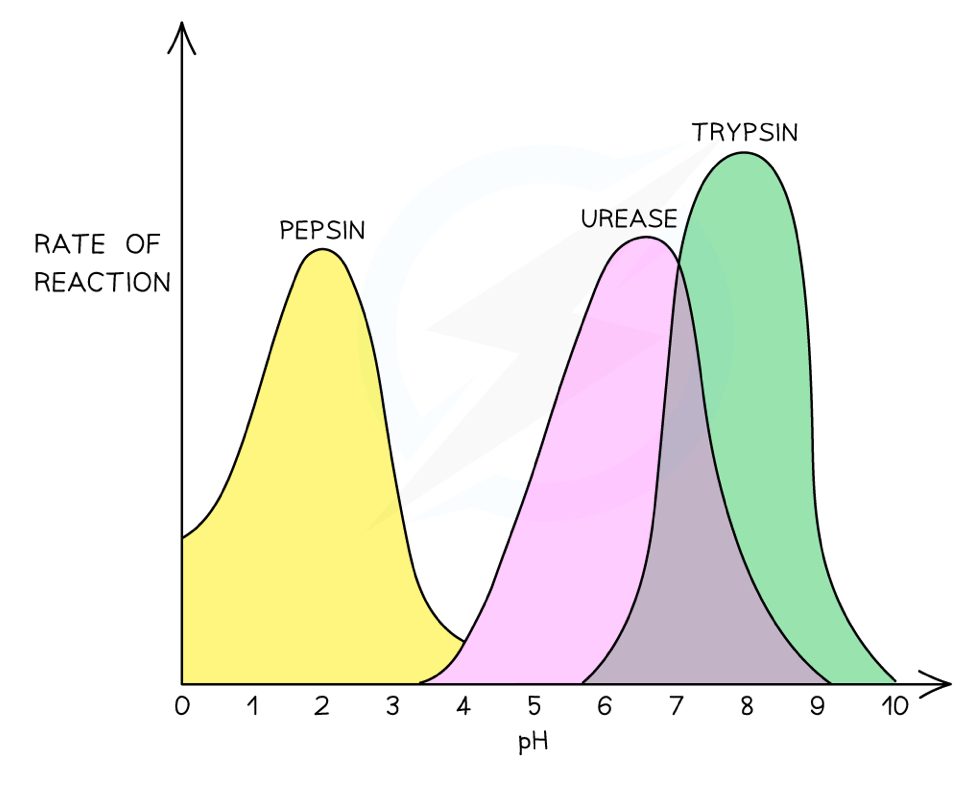
How does pH affect enzyme activity?
Hydrogen and ionic bonds hold the tertiary structure of enzymes together
At the optimum pH for an enzyme, it has the highest rate of reaction
Outside of an enzyme’s optimum pH, the H+ ions (acidic) and OH- ions (alkaline) can cause these bonds to break
This alters the shape of the active site, which means enzyme-substrate complexes form less easily, or not at all, where the enzyme has denatured
This can be demonstrated using amylase, starch solution, iodine and buffer solutions at different pHs
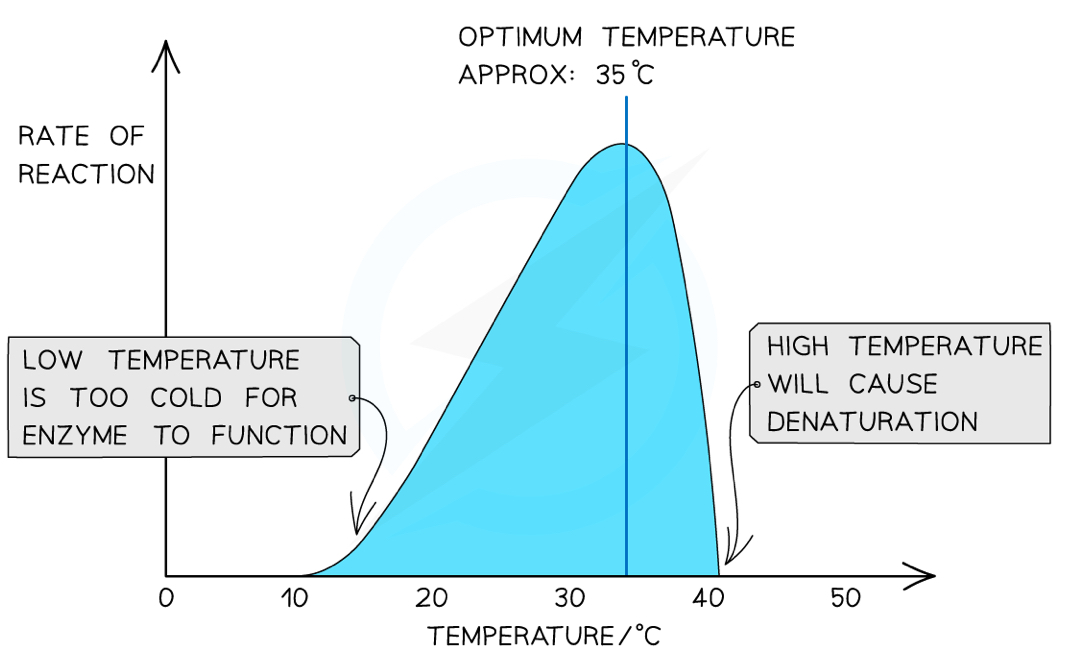
How does temperature affect enzyme activity?
At the optimum temperature for an enzyme, it has the highest rate of reaction
Below this temperature, the molecules have less kinetic energy and move slower, so they collide less often
Because they collide at lower temperatures, there is less activation energy, so there are fewer successful collisions
Above the optimum temperature, the particles have more kinetic energy and vibrate more, putting strain on the bonds and causing them to break
This changes the specific 3D tertiary structure of the enzyme, so it denatures
What is the temperature coefficient?
The temperature coefficient, Q₁₀, for a biological reaction measures how much the rate of reaction increases with a 10°C increase in temperature
Most enzymes have a Q₁₀ of 2

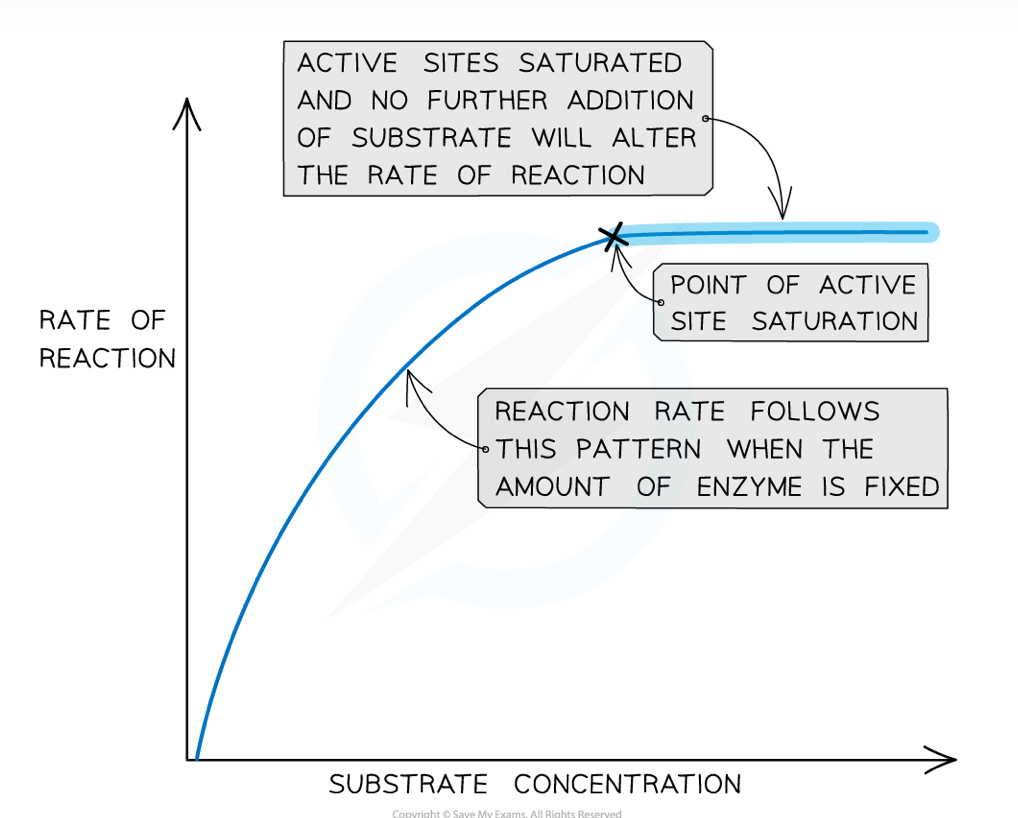
How do substrate concentration and enzyme concentration affect enzyme activity?
As enzyme or substrate concentration increases, rate of reaction increases
This is because there are more active sites available to form enzyme-substrate complexes in a given volume. so there is a higher collision rate
This continues up until the point of saturation, where the enzyme and substrate concentrations are the same and so all the active sites are filled
This point is the Vmax , where the rate of reaction is at its maximum possible - all active sites are occupied no more enzyme substrate complexes are formed
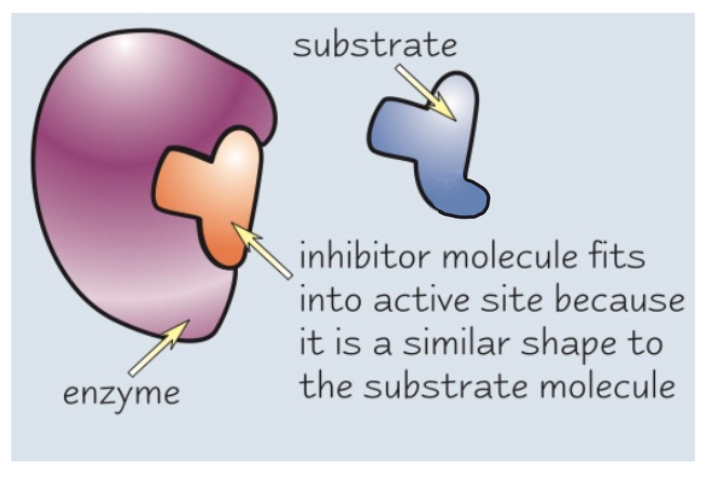
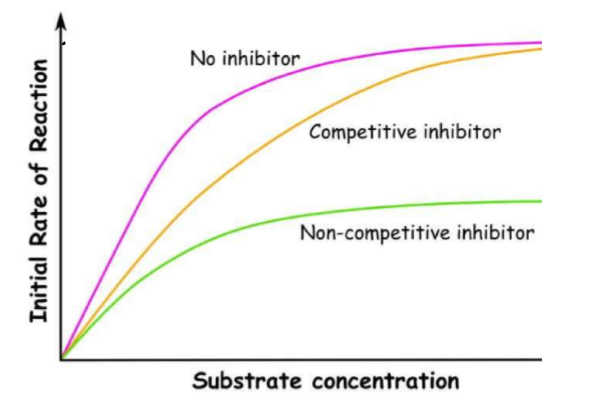
What do competitive inhibitors do?
Competitive inhibitors have a similar shape to the substrate
This means they can bind with the active site of an enzyme before the substrate can, saturating the active sites
The substrate then can’t bind and no reactions can occur (competing)
however the Vmax does not change
e.g. statins
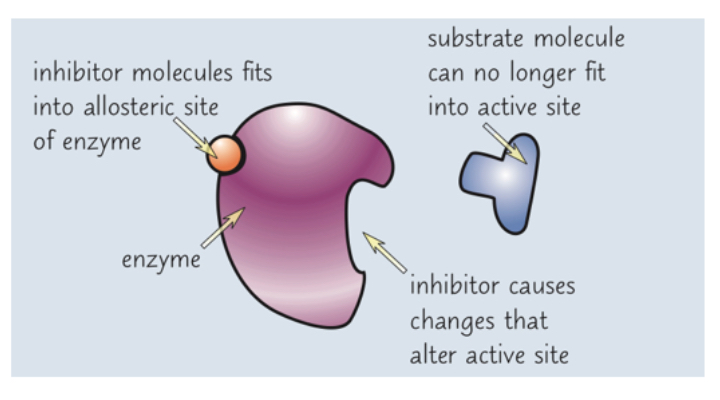
What do non-competitive inhibitors do?
Non-competitive inhibitors bind to the allosteric site, separate from the active site
This binding alters the 3D tertiary structure of the enzyme as a whole, including the active site
This change in shape mean the substrate can no longer bind so no reactions can occur
How can we compete with competitive inhibitors?
Increasing substrate concentration means the substrate is more likely to collide with an active site, so it can reach the normal V max
This doesn’t work for non-competitive inhibitors as they active site has changed shape
What are reversible and non-reversible inhibitors?
Reversible inhibitors only form weak ionic or hydrogen bonds with enzymes, so they can be removed (temporary)
Irreversible inhibitors form strong covalent bonds with enzymes, so they can’t be removed (permanent)
How can different inhibitors be used in medicine?
Different inhibitors can be used to treat diseases and conditions by inhibiting certain enzymes involved in them, eg.
PPIs can treat long-term indigestion, blocking an enzyme system responsible for secreting H ions into the stomach
How can different inhibitors act as metabolic poisons?
Non-reversible inhibitors permanently inactivate enzymes, which can completely stop a biological reaction- some of these inhibitors are used as metabolic poisons as they prevent vital processes, eg.
Cyanide is a non-reversible inhibitor of a mitochondrial enzyme in respiration
What is end-product inhibition?
When the product of an enzyme-controlled reaction or pathway inhibits the enzyme that produces it, controlling the process (negative feedback)
excess products are not made and resources aren’t wasted
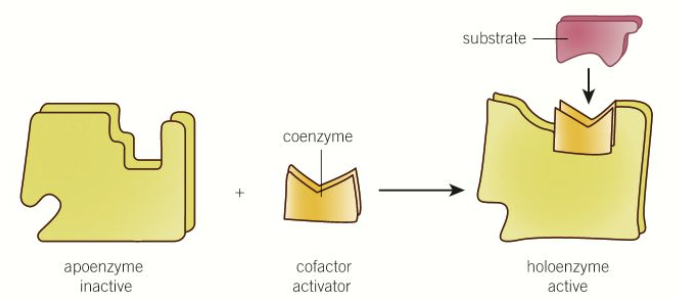
What are cofactors?
Some enzymes are inactive until they combine with another compound that changes their 3D tertiary structure in order to bind with the substrate correctly
They can be organic, inorganic or prosthetic groups
What are inorganic cofactors? Include an example
Some enzymes need inorganic ions to function, which are acquired from minerals
Eg. chloride ions act as a cofactor for amylase; in order to digest starch into maltose, chloride ions must be present
What are organic cofactors? Include an example
Larger organic cofactors are known as coenzymes
They can either temporarily or permanently bind to the enzyme
Coenzymes are involved in carrying electrons or chemical groups between enzymes, especially in a metabolic pathway
Coenzymes are derived from vitamins
Eg. vitamin B3 is used for NAD and NADP, coenzymes that are involved in respiration and photosynthesis respectively
What are prosthetic group cofactors? Include an example
Prosthetic groups are permanent cofactors, they are always bonded as part of the enzyme
Eg. zinc ions act as the prosthetic group for carbonic anhydrase, which metabolises carbon dioxide
what is precursor activation
used with enzymes which:
can cause damage within cells where they are released
where enzyme action needs to be controlled and only activated under certain conditions
need to undergo a change in shape, to be activated, can be done through the addition of a cofactor
before the cofactor - apoenzyme
after - holoenzyme
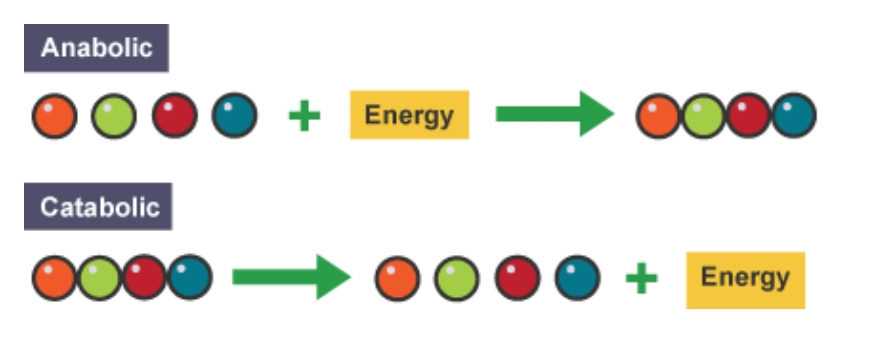
What are the two types of metabolic pathway?
Anabolic- builds up large molecules from smaller ones (exothermic)
Catabolic- breaks down large molecules into smaller ones (endothermic)
What are the 4 main biological roles of water?
Habitat
Solvent
Coolant
Transport medium
Why is water a polar molecule?
The oxygen atom attracts the electrons more strongly than the hydrogen atoms
This gives the oxygen a weak negative charge (δ-) and the hydrogen a weak positive charge (δ+)
This means water has a dipole
How do hydrogen bonds work in water and why are they useful?
Weak hydrogen bonds form between the hydrogen and oxygen atoms of adjacent water molecules, due to it’s polarity
This means that water:
Is a good solvent as it attracts other polar molecules
Has a high specific heat capacity + latent heat of vaporisation
Is less dense when it freezes
Has a high cohesion to itself and high surface tension
adhesive properties - water attracted to other materials
How do the properties of water relate to it’s role as a solvent, and what are the examples of it?
Water is a polar molecule, so it attracts other polar molecules and dissolves them
Eg. Water can carry mineral ions in plant xylem + blood plasma carries blood cells and other substances
How do the properties of water relate to it’s role as a coolant, and what are the examples of it?
Water has a high specific heat capacity, so it can take in a lot of energy before changing temperature
It also has a high latent heat of vaporisation, so it takes in a lot of energy when boiling
Eg. Evaporation is used to cool down, by sweating or panting
How do the properties of water relate to it’s role as a habitat, and what are the examples of it?
Water has a high specific heat capacity, so it can take in a lot of energy before changing temperature
Freezes in a crystalline structure, so it is less dense when solid, meaning ice floats and can insulate water bodies
Polar molecule, so it attracts other polar molecules and can dissolve them
Eg. Creates a stable environment in ponds, with a constant temperature (for enzyme activity) and dissolved nutrients
How do the properties of water relate to it’s role as a transport molecule, and what are the examples of it?
Water is a polar molecule, so it attracts other polar molecules and can dissolve them
This also means it has high cohesion to itself and adhesion to surfaces, so can easily flow
Eg. Water can carry mineral ions in plant xylem + blood plasma carries cells and dissolved substances
What chemical elements make up carbohydrates, lipids, proteins and nucleic acids?
C, H and O for carbohydrates
C, H and O for lipids
C, H, O, N and S for proteins
C, H, O, N and P for nucleic acids
What are the biological cations calcium, sodium, potassium, hydrogen and ammonium each used for?
Calcium (Ca 2+) - nerve impulse transmission and muscle contractions
Sodium (Na +) - nerve impulse transmission and kidney function
Potassium (K +) - nerve impulse transmission and stomatal opening
Hydrogen (H +) - catalysis of reactions and pH determination
Ammonium (NH4 +) - production of nitrate ions by bacteria
What are the biological anions nitrate, hydrogen carbonate, chloride, phosphate and hydroxide each used for?
Nitrate (NO3 -) - nitrogen supply to plants for amino acid and protein formation
Hydrogen carbonate (HCO3 -) - maintains blood pH
Chloride (Cl -) - balance sodium and potassium ions in cells
Phosphate (PO4 3-) - cell membrane formation and bone formation and nucleic acid and ATP formation
Hydroxide (OH -) - catalysis of reactions and pH determination
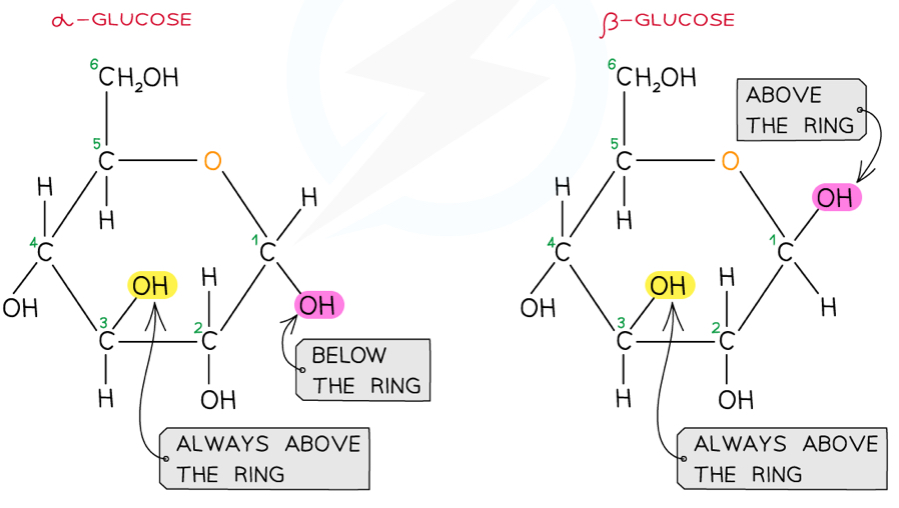
What are the structures of alpha and beta glucose?
Glucose is a hexose sugar with two isomers- they both have the formula C6H12O6
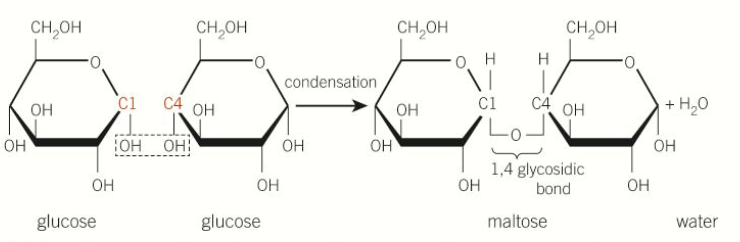
describe the condensation reactions between glucose molecules
2 hydroxyl groups react, new bonds are reformed
between alpha glucose a 1,4 glycosidic bond forms
one H2O is produced
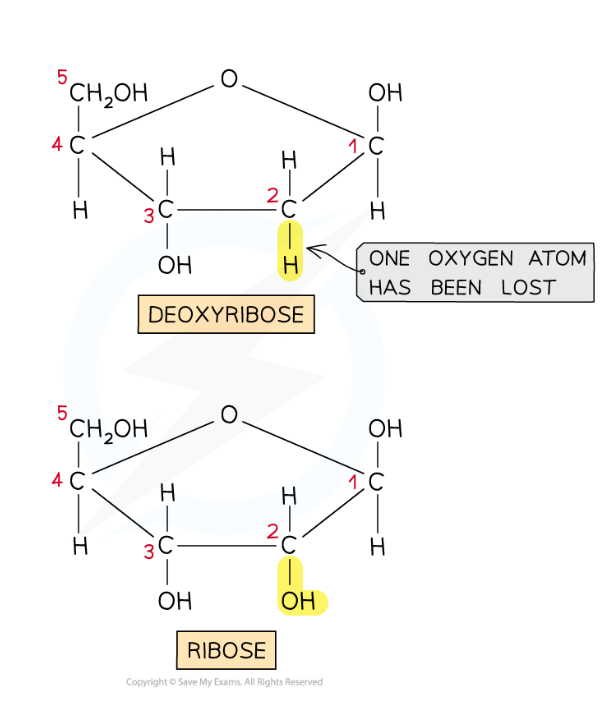
What are the structures of ribose and deoxyribose?
Ribose and deoxyribose are pentose (5 carbon) sugars, with similar formulas except that deoxyribose has one less oxygen than ribose (lost from the second carbon)
What three properties do monosaccharides have in common?
Soluble in water
Sweet tasting
Forms crystals
How can disaccharides and polysaccharides be formed and broken down?
They can be formed by condensation reactions- when two hydroxyl (OH) groups from different saccharides interact to produce a water molecule and a glycosidic bond between the two saccharides
This can be catalysed by enzymes
They can be broken down by hydrolysis- when water is added to a di or polysaccharide, breaking the glycosidic bond to form a hydroxyl group on each saccharide
This can be catalysed by (different) enzymes
We use this to test for non reducing sugars
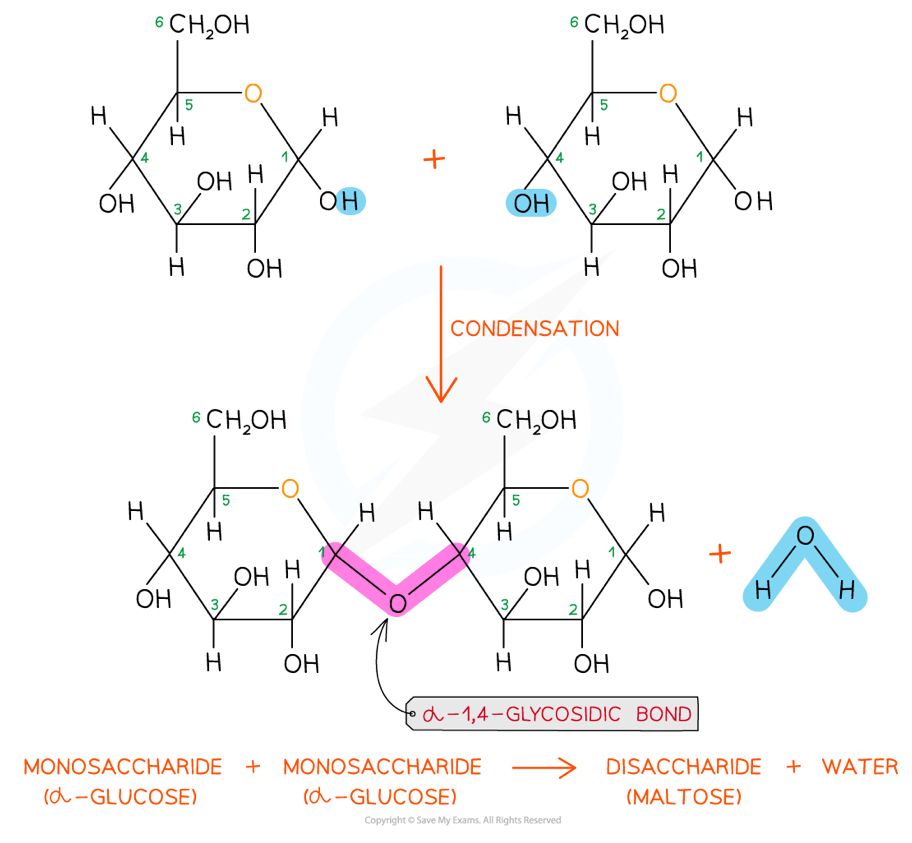
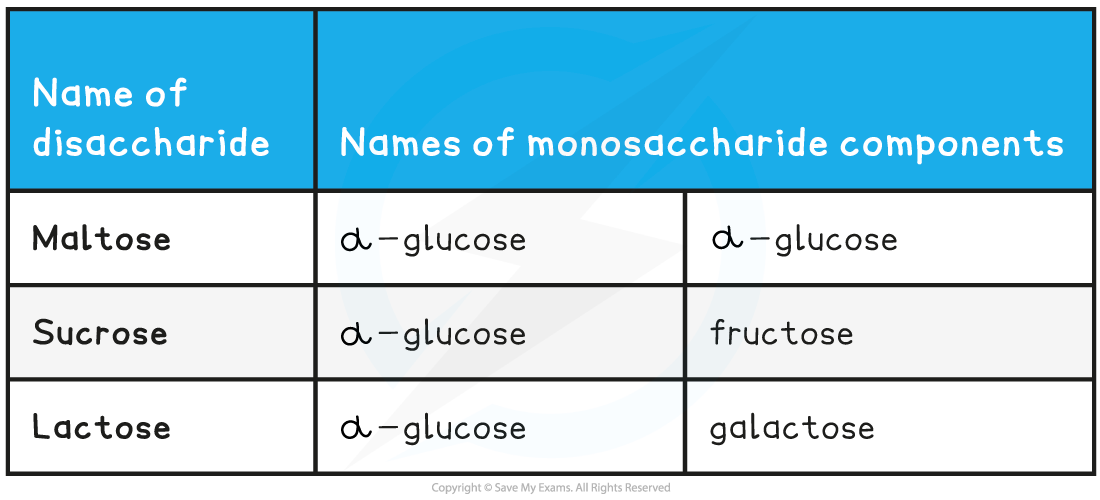
What are the three most common disaccharides made from?
Maltose- two alpha glucose molecules
Sucrose- glucose + fructose
Lactose- glucose + galactose
What are reducing sugars?
Reducing sugars can give away electrons via the oxidisation of a carbonyl (C=O) group
This is why reducing sugars can be detected using Benedict’s solution- they reduce the soluble blue copper sulphate to insoluble brick-red copper oxide
All monosaccharides and some disaccharides are reducing sugars- polysaccharides aren’t
How can we detect non-reducing sugars and why?
Non-reducing sugars (di or poly saccharides) must be broken down into their monosaccharides, which are always reducing sugars, to be detected using Benedict’s solution
We do this by hydrolysis, where we heat the sample with hydrochloric acid to break the glycosidic bond, and then neutralise it
Then we can test with Benedict’s solution to see whether reducing sugars were produced, and hence whether non reducing sugars were originally present
What is the structure of starch?
Starch is made from two different alpha glucose structures :
Amylose (20%)- a straight chain linked by 1,4-glycosidic bonds- amylose curls into a helix shape which allows it to be more compact
Amylopectin (80%)- a branched chain linked by 1,4 and 1,6-glycosidic bonds
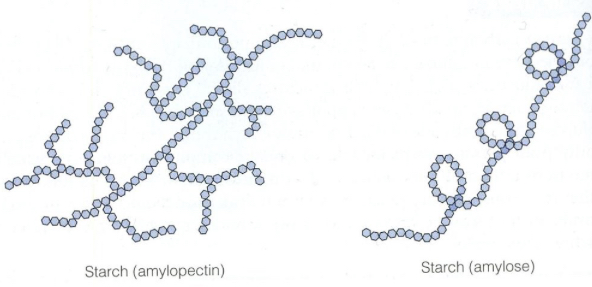
What is starch used for and how is it well suited?
Starch is the main carbohydrate store in plants
Stored in the plastids- amyloplasts and chloroplasts
This because it is:
Compact, so large quantities can be stored
Insoluble, so it won’t change the water concentration in cells and affect osmosis
Amylopectin (80%) is linked by some 1,6-glycosidic bonds, so it has many terminal glucose molecules that can be hydrolysed for respiration or added for storage
What is the structure of glycogen?
Made up of alpha glucose molecules linked by 1,6 and 1,4-glycosidic bonds
Glycogen has a similar structure to amylopectin but is more branched, because it has more 1,6-bonds
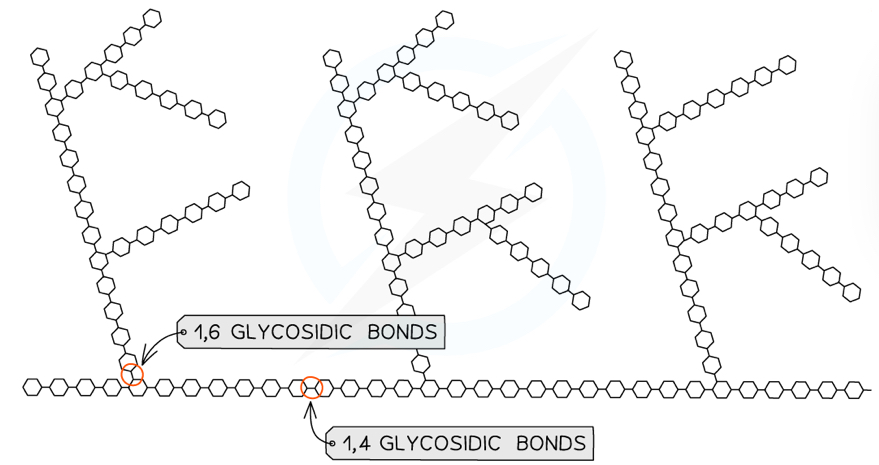
What is glycogen used for and how is it well suited?
Glycogen is used for storage in animals
Stored in liver and muscle cells
This because it is:
Compact but relatively large, so large quantities can be stored (more 1,6-bonds means it is more compact than amylopectin)
Insoluble, so it won’t change the water concentration in cells and affect osmosis
Linked by many 1,6-glycosidic bonds so it has many terminal glucose molecules that can be hydrolysed for respiration or added for storage
What is the structure of cellulose?
Made up of beta glucose molecules linked by 1,4-glycosidic bonds
To bond together, every other beta glucose molecule is flipped
This means that hydrogen bonds can form between strands, to create microfibrils
These make up the cellulose fibres that link into a network
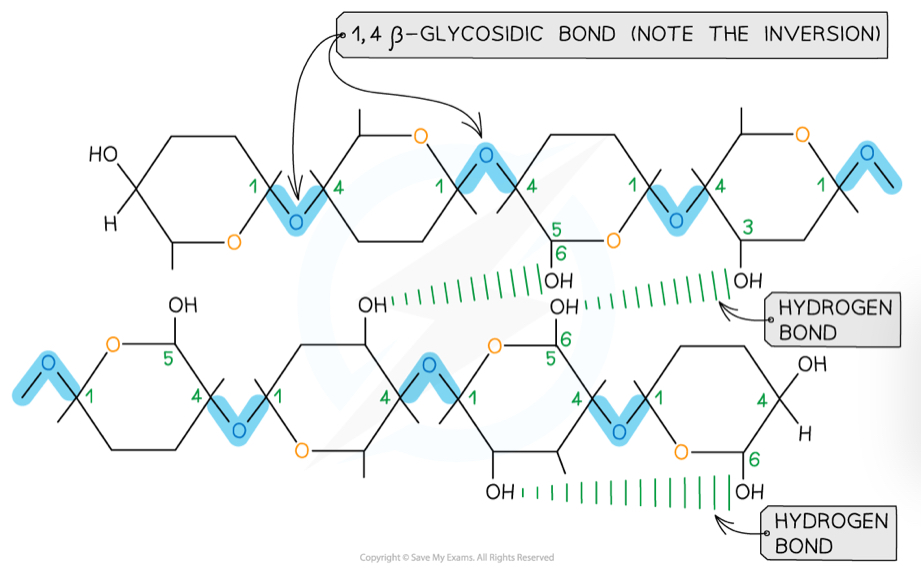
What is cellulose used for and how is it well suited?
Cellulose makes up the majority of plant cell walls
This is because it is:
Held together by many hydrogen bonds between strands, so it has a very high tensile strength and is able to withstand the pressure from turgidity of the cell
Linked to other molecules like lignin, which increases the strength of the cell walls
Permeable, so water and solutes can enter or leave the cell