Colloids and suspensions
1/135
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
136 Terms
What type of free energy does a system favour?
Lowest free energy = least interaction between 2 phases = least shared SA
What is a disperse system? What are the 2 phases?
2 or more components in a heterogeneous mixture
Disperse and Continuous phase
What is a formulation with a liquid dispersed and liquid continuous phase?
Emulsion
What is a formulation with a solid dispersed and solid continuous phase?
Suppositories
What size are colloidal dispersions?
Particles 1nm-1μm in diameter
Not visible to naked eye
How to tell difference between colloid dispersion and solution? What is the effect called?
Dispersion will scatter light = illuminating path of beam of light
Tyndall effect
Difference between colloidal and coarse dispersions?
Colloidal suspensions have smaller particles (<1μm diameter) so gravity does not exert enough of an effect on them to cause settling
How do particles move in colloidal dispersions?
Brownian motion to diffuse within continuous phase
How can colloidal dispersions be separated?
Ultracentrifugation
Examples of daily colloidal dispersions?
milk, printing ink, blood
(plasma proteins rather than RBCs which will settle with time- erythrocyte sedimentation rate [ESR] test)
What is a suspension? How big are particels?
a coarse dispersion
Particles >1μm in diameter
What is the effect of gravitational forces on coarse dispersions/suspensions?
Larger particles (>1μm diameter) so gravity causes then to settle upon standing
How does the speed of sedimentation affect dosing?
Must be slow enough that a homogeneous dose can be obtained (poured/drawn into syringe) following shaking before settling again
May take some patients longer to dose e.g. arthritis
Can suspensions be delivered parenterally? Why?
Not usually as capillary diameter is 8-10μm so if particles (>1μm diameter) clump together they can be too large to enter caps
Advantages of dispersed systems (degradation, absorption x2, adsorption, taste)
Drugs susceptible to aqueous degradation may be formulated as a suspension
Increased absorption speed compared to solid oral dosage forms due to small particle size – slower than solutions though
Particle size can be tailored to optimise absorption rates
Active has high SA which can be used for adsorption (e.g. kaolin used for adsorption of toxins in GI tract for food poising)
Allows flexibility of formulation e.g. better taste masking of bitter drugs
Disadvantages of dispersed systems (liquids: stability, packaging; dosing)
As for all liquid formulations:
Active/excipient stability problems in presence of liquid
Liquids (esp. aqueous) are susceptible to microbial contamination
Administration of the correct dose can be less precise – settling, may take some patients longer to pour medicines (e.g. elderly with arthritis)
Size/weight/packaging issues for liquid formulations
More difficult to mask the taste of a drug in a liquid than solid formulation (although it is easier in a suspension than a solution)
If not shaken properly before each use, accurate dosing cannot be assured
How does particle size affect taste of drugs?
Particles > 10μm cannot enter taste bud pore so particles in suspension have little taste (even less in solid formulations) compared to suspensions
But usually all oral formulations require excipients to improve taste - sweeteners, flavourings
What are the ideal properties of a dispersed system?
Particles should be evenly distributed in the continuous phase
Particles must not settle/sediment too rapidly
Sediment must be easily re-dispersed
Must easily flow out of the container
Particle size should be both small and uniform
What is a lyophobic colloid? What does this cause?
Solvent ‘hating’ system
Particles have different characteristics to dispersant = little attraction to dispersant = thermodynamically unstable = colloidal particles will aggregate to lower surface energy
Examples of lyophobic colloids in water?
water insoluble drugs, clays, oils, inorganic particles
What is a lyophilic colloid? What does this cause?
Solvent ‘loving’ system
Particles have an affinity for the dispersant = particles interact with dispersant molecules = causes solvation (called hydration in water) = prevents coagulation = inherently stable dispersion
Examples of lyophilic colloids in water
starch
gums - acacia, tragacanth
What is an amphiphilic colloids
Molecules with regions of different affinities to continuous phase
Orientate so each part of molecule is in contact with preferred phase
How do molecules arrange in amphiphilic colloids?
Start by arranging at the surface of the continuous phase until reaching critical micelle concentration (conc at which no space left at surface)
Then amphiphiles form spherical structures called micelles in continuous phase to remain orientated to preferred phase
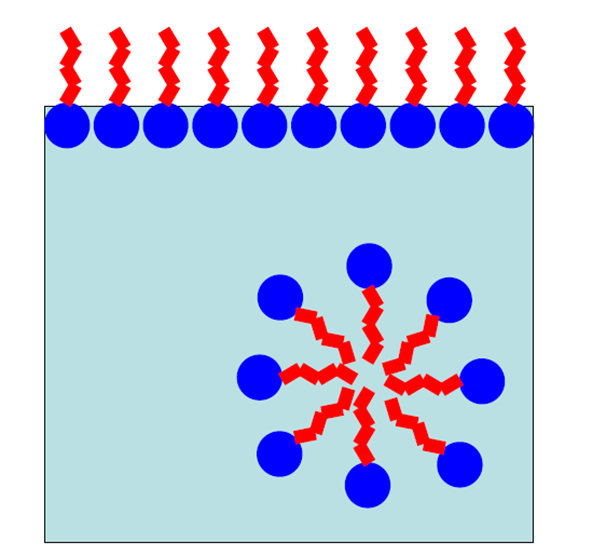
What is the critical micelle concentration?
Conc at which no space left for molecules at surface of continuous phase
Above this micelles will form
What are liposomes? advantages? examples?
Bilayer structure of amphiphiles
Have a ‘pocket’ of continuous phase within them which can be used to encapsulate lyophilic materials to control delivery - protect them from enzymatic degradation, can control delivery time
e.g. mRNA vaccine delivery systems (as mRNA unstable in body as body breaks down foreign/unprotected genetic material)
How can lyophobic drugs be ‘solubilised’ using amphiphilic molecules?
Lyophobic drugs can be dissolved within the middle of the micelle or the bilayer of the liposome – this is a means of utilising colloidal systems to ‘solubilise’ poorly soluble drugs
So they are protected from enzymatic degradation and delivery time is controlled
What are association colloids?
Micelles and liposomes
As while they are sort of colloidal systems they are not static structures
The amphiphilic building blocks constantly disassociate and reassociate with one another to form new structures
What are nanoparticles?
Colloidal systems with permanent solid structures as the dispersed phase that are 1-100nm
What 4 factors of nanoparticles can be changed to maximise usefulness?
Size can affect where on body drug acts
Surface by attaching molecules/fgs/charges
Shape
Material nanoparticles are made of
What size particles in colloids bend light more?
Smaller particles bend light more = higher degree of light scattering
What is the Tyndall effect? What are the results in solution, colloids and suspensions?
Shine beam of light through:
Solution = no beam visible
Colloid = scattering of light causes beam path to be illuminated
Suspensions = scattering of light causes beam path to be illuminated and individual particles to be seen
What degree of light scattering do different colour wavelengths have?
Long wavelength (red) = least scattered
Short wavelength (blue) = most scattered/bent
How can particle size in a dispersion be measured?
Using light scattering property:
A laser diffraction particle size analyser shines a beam of laser light onto particles in a single file stream (passed in a narrow tube)
A detector measures the amount of deflection of the light and from this calculates the size of a particle
Over time, the distribution of sizes amongst all of the particles in a sample are measured
Why aren’t all suspensions colloids?
Difficult to generate small enough particles
Surface energy causes them to flocculate (associate into larger clumps)
Wetting (solvation/hydration) of particles more difficult as air adsorbs to surface of smaller particle easier and will be more difficult to displace
What is wetting? what does it prevent (3)?
The solvation/hydration of a solid particle
Its ability to stay in contact with a liquid
First stage of making a suspension to prevent particles:
remaining on liquid surface
attaching to container
forming larger clumps = flocculation
Why are smaller particles more difficult to wet?
As have adsorbed layer of air around particles that is harder to displace
What type of solids need a wetting agent?
Indiffusible solids
As they show some hydrophobicity so are not easily wetted
What is the purpose of a wetting agent?
They decrease interfacial tension to allow wetting and help dispersion
What are the forces that exist between the molecules in each phase called?
Cohesive forces
What are the forces that exist between the two phases called?
Adhesive forces
Are cohesive or adhesive forces usually greater? What does this cause?
Cohesive forces (between molecules of same phase) are usually greater
Causing tension (imbalance of forces) at the interface
How is wettability determined?
The degree of wetting is determined by the difference between adhesive (different phases) and cohesive (same phase) forces?
What is the main type of wetting agent? How do they work? What does shaking do (2)?
Surface Active Agents (SAAs - detergents)
Hydrocarbon chains that are adsorbed to hydrophobic particle surfaces while polar groups enter the liquid and become hydrated.
Shaking causes:
excessive foaming - due to stabilisation of bubbles created when air is mixed into the system
deflocculation of the system
How can hydrophilic colloids be used as a wetting agent?
Used in combination with SAA to cause deflocculation
Coat the solid hydrophobic particles with a layer, giving the particle a hydrophilic character
How can solvents be used as wetting agents?
Water miscible solvents decrease interfacial tension by:
penetrating powder clumps
displacing air
What is the effect of gravity on Brownian motion?
Brownian motion keeps small particles moving in a colloidal dispersion but as the particles get bigger, the influence of gravity is greater
Particles >0.5 -1μm start to have more significant gravity and will start to settle to the bottom
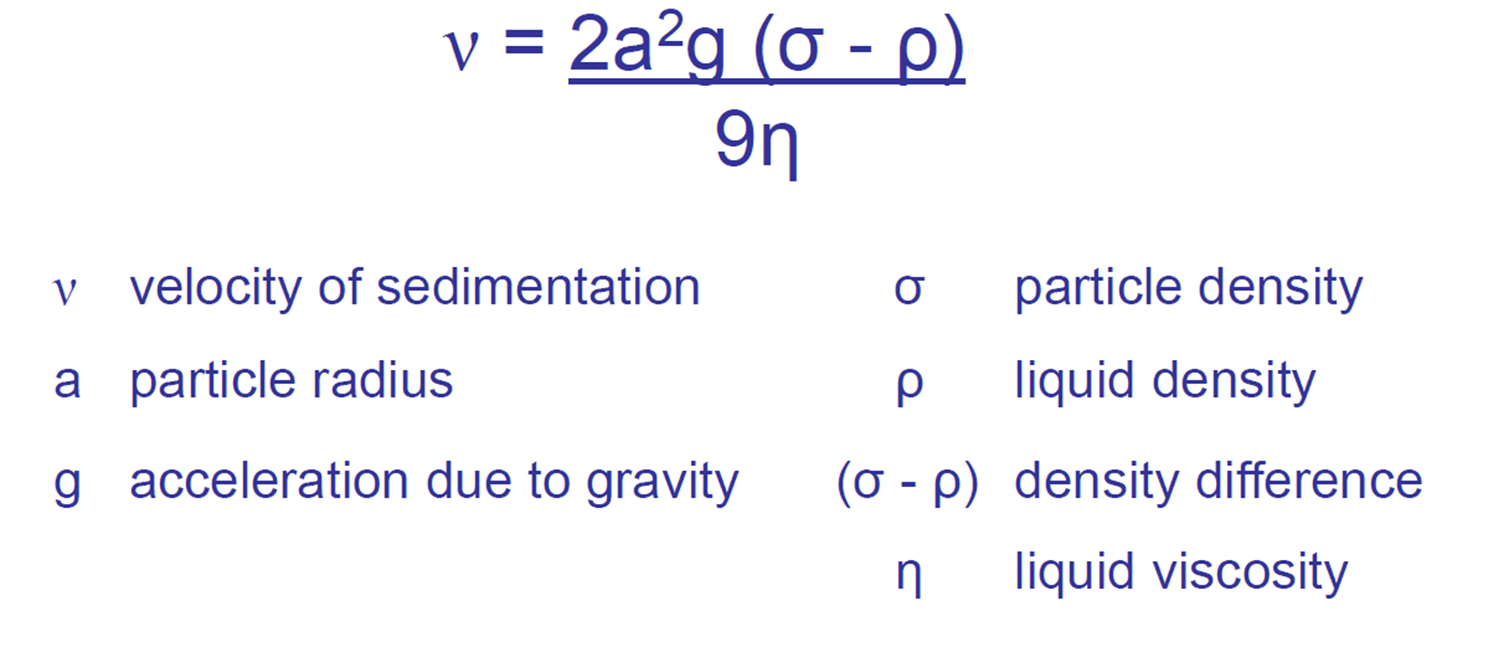
What is the idea of stokes law?
Velocity of sedimentation:
decreases as liquid density increases
increases as particle radius/g/density difference increases
What is Ostwald ripening?
Small particles dissolving in solution at higher temperature
Dissolved particles will join onto surface of larger particles when temp decreases and saturation is reached
Causes small particles to shrink and disappear, while large particles grow
Leading to much larger particles that will settle more quickly
When does Ostwald ripening occur?
When suspensions have particles of different sizes
Why do larger particles sediment quicker?
More mass = higher acceleration due to gravity (g)
What measures can be taken to prevent Ostwald ripening when dispersed phase has borderline soulbitly?
Lower temperature
Antisolvents
pH change
Drug salts created that are poorly soluble in continuous phase
Does the abosulte density value of particle/vehicle affect sedimentation rate?
No
The density difference between the two phases is important
What are density modifiers used for? Examples?
Tend to increase density of continuous phase
To lessen density difference
Syrup, glycerol, propylene glycol
What is viscosity? in terms of intermolecular forces?
The internal friction of a fluid, produced by intermolecular forces within the liquid phase
What factors affect viscosity?
Molecular size
Molecular shape
Molecular chemistry
Molecular concentration
Temperature
How does molecular size affect viscosity?
Larger molecule = higher viscosity
How does molecular shape affect viscosity? How does branching affect viscosity?
Shape influences area over which intermolecular forces occur
More branched = less points of contact for IMF = less IMF = less viscous
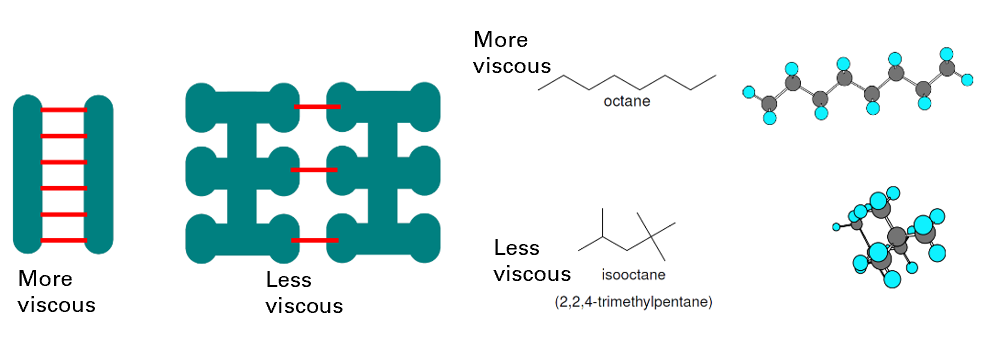
How does molecular chemistry affect viscosity? How does polarity affect viscosity?
Polar molecules can form hydrogen bonds = creating resistance to movement = increasing viscosity
How does molecular concentration affect viscosity?
Higher conc = more interactions between molecules = higher viscosity
How does temperature affect viscosity?
increase temp = adding energy to system = more movement of molecules and breakage of intermolecular interactions = decreases viscosity
What is rheology?
The study of flow
shearing stress and rate of shear
What is the shearing stress of a liquid?
The force per unit area required to cause the liquid to flow is called the “shearing stress” (σ),
σ = F/A
What is the rate of shear of a liquid?
The difference in velocity (dv) between two planes of liquid a given distance apart (dx) is the “rate of shear” (γ)
γ = dv/dx
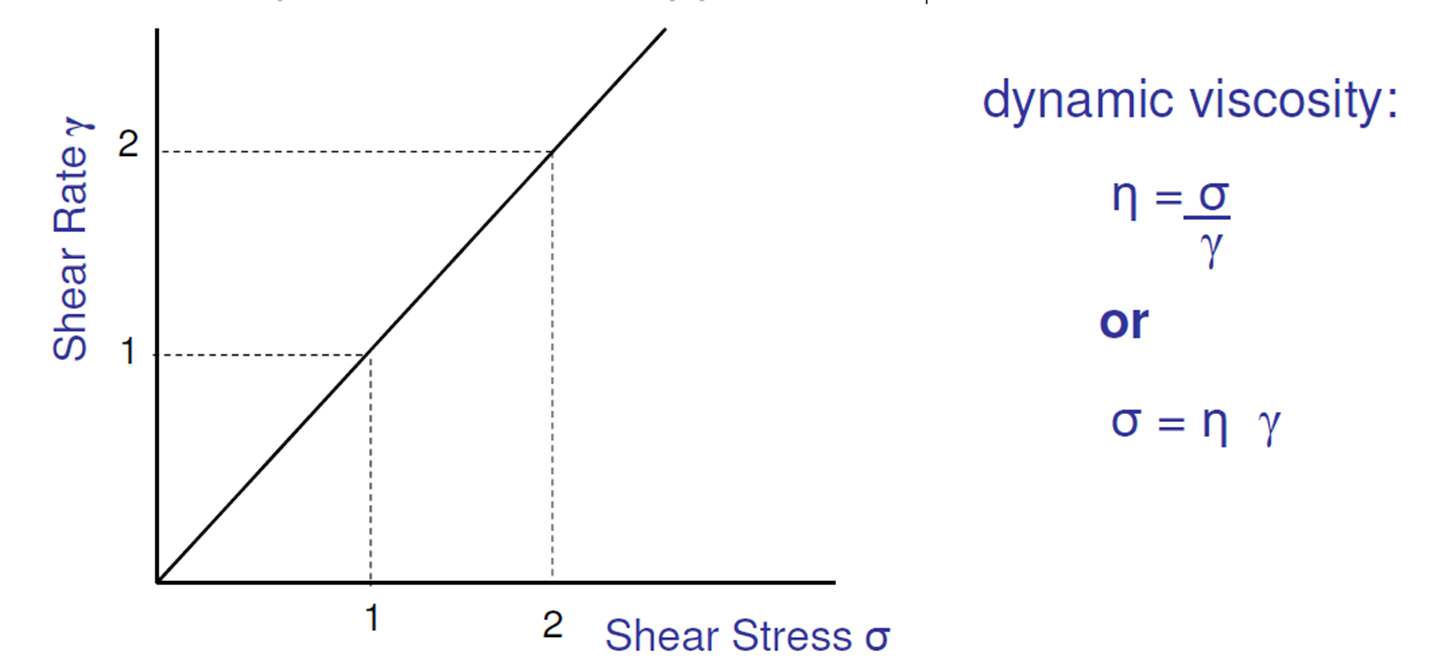
What is the main relationship between shear stress and rate of shear?
Directly proportional for Newtonian fluids
How does viscosity affect shear stress?
The higher the viscosity of a liquid,
The greater the force per unit area (shear stress)
required to produce a given flow rate
What is a Newtonian fluid?
Substances in which shear rate is directly proportional to shear stress
They continue to flow in the same way, regardless of the forces acting on them
Their behaviour is simple and predictable, they behave as expected when disturbed or agitated
When pushed or pulled, they move or change shape in proportion to the force applied
Is water a Newtonian fluid? Why?
Yes
continues to behave in same way regardless of how fast is stirred/mixed
it has a proportional increase to the force of flow
What is Stoke’s law formula?
What is dynamic viscosity of a Newtonian fluid?
shear stress/shear rate
(inverse of gradient)
What is a non-Newtonian substance?
Do not have a constant viscosity
Viscosity changes with the applied shear force
Properties change in response to movement and deformation
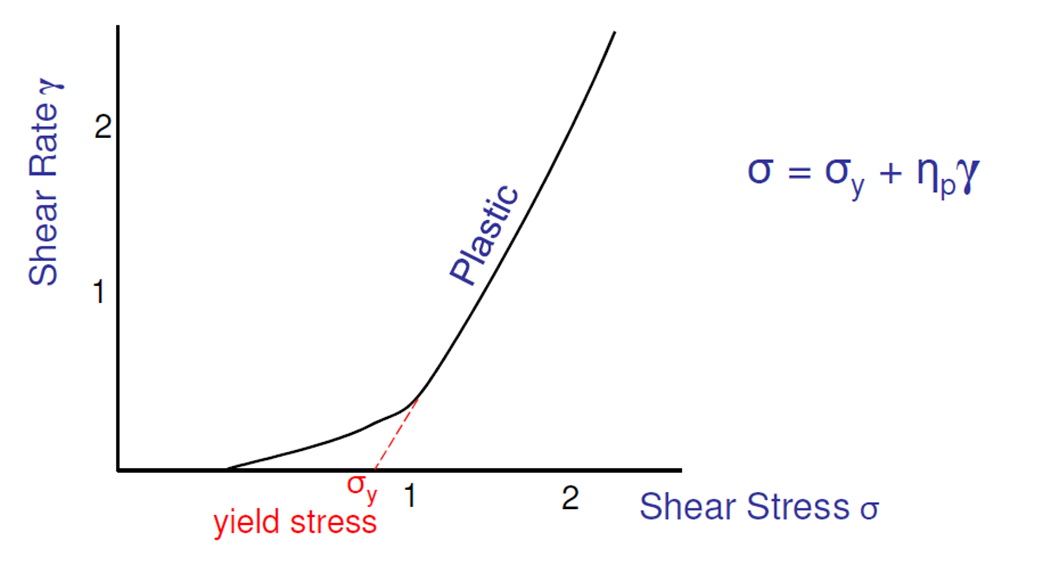
What is plastic flow? what suspensions is it seen in?
A minimum force beyond which the material flows (yield stress/value)
Seen in concentrated suspensions with flocculated particles and high viscosity continuous phases
(as won’t flow much initially until a greater force is applied because acting quite solid-like)
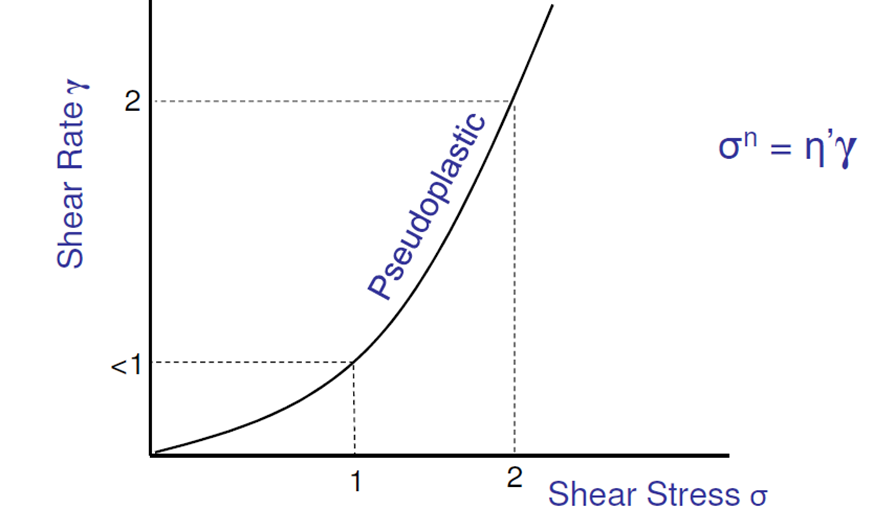
What is pseudoplastic flow? What type of suspension is it seen in?
Viscosity decreases with the rate of shear = “shear thinning” fluid
Seen in flocculated dispersions containing long, high molecular weight molecules
The more stirring, the thinner the liquid gets, the more it flows: in-plane alignment, facilitates easier slipping past each other
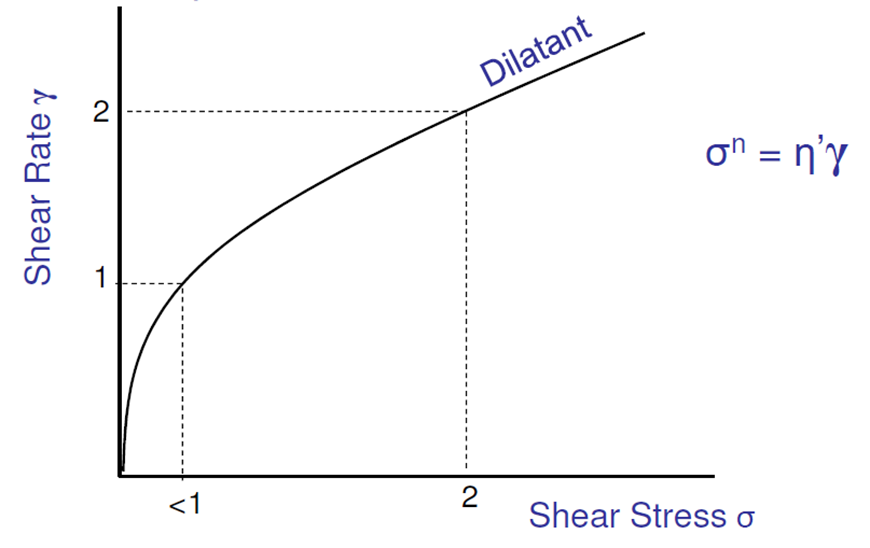
What is dilatant flow? what type of suspension is it seen in?
Viscosity increases with the rate of shear = “shear thickening” fluid
Dispersions containing a high concentration of small, deflocculated particles
The more stirring, the thicker the liquid: out of plane alignment/aggregation, preventing slipping
How does shear thinning happen at a molecular level (alignment)?
in-plane alignment, facilitates easier slipping past each other
How does shear thickening happen at a molecular level (alignment)?
out of plane alignment or aggregation, preventing slipping
What is thixotropy?
Reversible, time-dependent change in viscosity, in some non-Newtonian fluids
At rest, particles form loose 3D gel structure
Shear stress via shaking disrupts bonds = causes shear thinning
When applied stress stops, the downcurve is displaced to the left indicating the gel structure reforms, but not immediately
For a given shear stress (X) the shear rate is lower (thicker) on the upward curve than the downward curve (thinner)
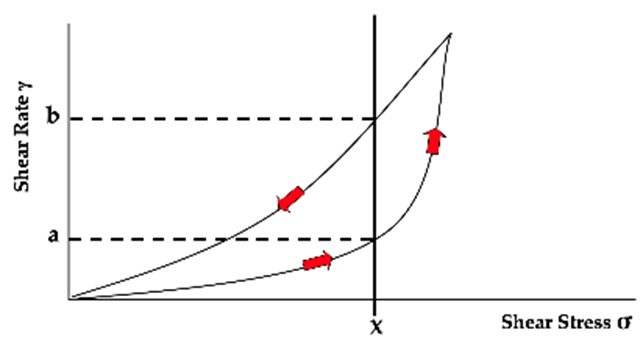
In thixotropy, for a given shear stress, is the rate higher on the downcurve or upcurve?
Downcurve is to the left
Shear rate is higher on the downcurve
Meaning viscosity (stress/rate) is lower = it is thinner
When does rheology matter in manufacturing and patient considerations?
Manufacturing considerations:
Mixing, Passing through machinery, Pouring/packing bottles
Patient considerations:
Physical stability
Ease of use e.g. pouring / spreading / injecting
Safety e.g. consistent dosing
Is a more or less viscous liquid preferred in storage? What type of shear stress is storage?
Storage = low shear
High viscosity preferred to slow/reduce sedimentation
Is a more or less viscous liquid preferred when shaking? What type of shear stress is shaking? (shaking prior to dosing)
Shaking = high shear
Low viscosity preferred to redisperse particles
What is the preferred flow for suspensions?
Pseudoplastic flow (and thixotropic)
high viscosity at low stress, low viscosity at high stress
slower sedimentation, faster redispersion
low gradient at low stress, high grad at high stress
Give examples of viscosity modifiers
polysaccharides, water-soluble celluloses, hydrated silicates, carboxypolymethylene, colloidal silicon dioxide
What are some pros/cons of natural products vs synthetic excipients?
Natural products are cheaper
Natural products suffer more from batch-batch variation
natural products are more of a risk factor for spore/microbial contamination so need more preservatives or shorter shelf-life
How are polysaccharides used as viscosity modifiers? examples? pros/cons (uses, pH, natural/synthetic/efficacy)?
Tragacanth, alginates, acacia gum, xanthan gum (Keltrol), starch
They form viscous thixotropic, pseudoplastic preparations
Can be used internally or externally - but sometimes feel too sticky topically
Narrow pH stability range
Mainly natural products: short-shelf life/contamination, batch-batch variation
Acacia and starch are not viscous enough alone so often combination with tragacanth
How are water-soluble celluloses used as viscosity modifiers? examples? pros/cons (viscosity, pH, natural/synthetic, efficacy)?
sodium carboxymethylcellulose, methylcellulose, hydroxyethylcellulose, microcrystalline cellulose
Semisynthetic polysaccharides (form viscous thixotropic, pseudoplastic preparations) with varying chain lengths
Longer chains = increased viscosity
Stable over wide pH range (unlike psc)
Some are non-ionic so suitable for use with ionic additives
Semisynthetic: less batch-batch variation, less contamination, more expensive
As more viscous than polysaccharides most are suitable to be used alone, combination not needed unlike acacia and starch psc
How are hydrated silicates used as viscosity modifiers? examples? pros/cons (uses, pH, natural/synthetic, efficacy)?
bentonite, hectorite, magnesium, aluminium silicate (Veegum)
Clays mined from ground = natural product
Used for both internal and external medicines (despite source)
Readily absorb up to 12x own mass of water = form thixotropic gels
natural products: cheaper, short-shelf life/contamination, batch-batch variation
How is carboxypolymethylene used as a viscosity modifer? pros/cons (uses, synthetic/natural, pH)
Synthetic polymer: less batch-batch variation, less contamination, more expensive
External use
Forms high viscosity products between pH 6-11 (alkaline) - very thin outside range
How is colloidal silicon dioxide used as a viscosity modifier? pros/cons (suspension use, synthetic/natural)
Synthetic: less batch-batch variation, less contamination
Aggregates in water to form 3D network (thixotropic)
Can be used in non-aq suspension
What is dynamic viscosity? units?
For a Newtonian liquid, the rate of flow (shear, γ) is directly proportional to the applied force (stress, σ)
The coefficient that describes the relationship is the dynamic viscosity (η):
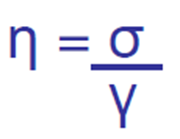
σ = force / area = N/m2 (or Nm-2, or Pascal [Pa])
γ = change in velocity / change in distance
= ms-1/m which cancels to s-1
η = σ / γ
η = Nm-2/s-1 (or Nm-2s, or Pa.s)
What is kinematic viscosity? units?
Relates a liquid’s viscosity to its inertial force – inertial force is equivalent to density (ρ)
ρ = mass (kg) / volume (m3)
(note: 1 m3 = 1000 litres)
The coefficient that describes the relationship between dynamic viscosity (η) and density/inertial force (ρ) is kinematic viscosity (ν):
•ν = η / ρ = dynamic viscosity/density
•Units of ν are m2s-1
(1 m2s-1 = 1,000,000 mm2s-1)
What are the 3 methods of measuring viscosity?
Capillary methods - how fast sample flows
Rotating methods - how hard it is to stir sample
Falling methods - how long it takes something to fall through sample
What are the 3 ways surfaces acquire an electrical charge when they come in contact with water?
Ion dissolution
Ionisation
Ion adsorption
How does ion dissolution affect surface charge?
Placing solid particles into water
More soluble ions dissolve into bulk of water
Leaving less soluble ions behind to create the particle surface charge
e.g. AgI:
Ag+ is more soluble in water so dissolves
Leaving I- ions behind so solid particle surface has a net negative charge
How does ionisation affect surface charge when pH is >/</= pKa?
Molecules at solid surface become ionised so particles acquire a surface charge
pH > pKa = negative charge particle surface - as alkaline solution takes cations, so cations are lost/anions are gained
pH < pKa = positive charge particle surface - as surface gains cations (e.g. H+ from acidic environment)
No net charge at isoelectric point = Zwitterion
What surface charge will ionisable particles have when pH > pKa?
negative
as alkaline solution so anions gained by particle
How does ion adsorption affect surface charge of particles? What is the usually surface charge of particles in water?
A surface charge can be acquired by the unequal adsorption of ions onto the surface
if more anions adsorb to a surface, the surface will develop a net negative charge.
Solid particle surfaces in water are more often negatively charged
because cations are generally more hydrated than anions
so cations tend to stay in the water phase
leaving the anions free to adsorb to the surface
This can involve OH- / H3O+ ions in water
How are ions distributed in dispersed systems? What is their charge if they have a surface charge?
A disperse system is overall electrically neutral and distribution of ions is uniform, but the surface charge of the particles influences the distribution of ions in the rest of the liquid
There is a layer around each particle with a different composition from the rest of system known as the electrical double layer and can be split into:
The inner region: includes the charged surface & adsorbed ions
The diffuse region: lies beyond adsorbed ions and up to the edge of the electrically neutral region
The electrically neutral region: outside the EDL (the bulk continuous phase)
What is the electrical double layer? What lies beyond it? stern and shear plane?
The inner region: includes the charged surface & adsorbed ions
stern plane is within inner region, particles not free to move
shear plane is between inner and diffuse region, beyond it particles move freely
The diffuse region: lies beyond adsorbed ions and up to the edge of the electrically neutral region
initially opposite charged counterions, then some co-ions moving further away
The electrically neutral region: outside the EDL = the bulk continuous phase
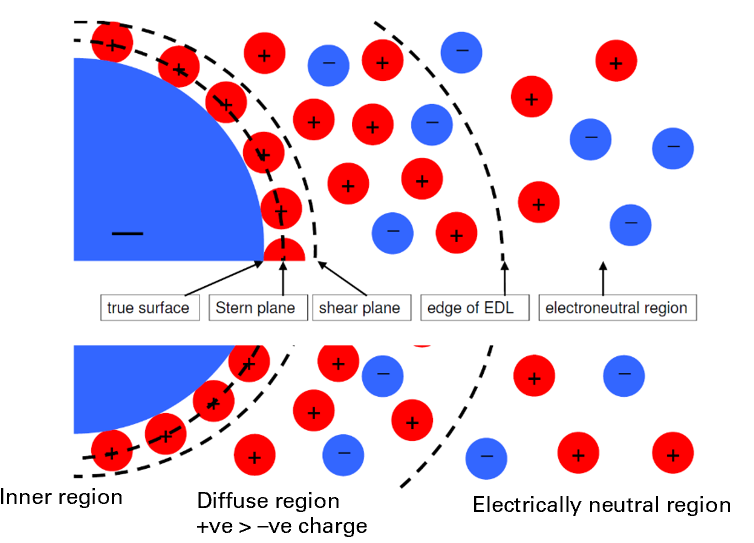
What causes uneven distribution of ions in a disperse system?
the electrical double layer
some areas with more charge than others
potential = charge difference