Sn1 Reaction Lab
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
What is the difference between “traditional” Wiliamson ether synthesis and reaction which you will perform?
Uses tertiary alkyl halide which makes this reaction an SN1 as opposed to SN2
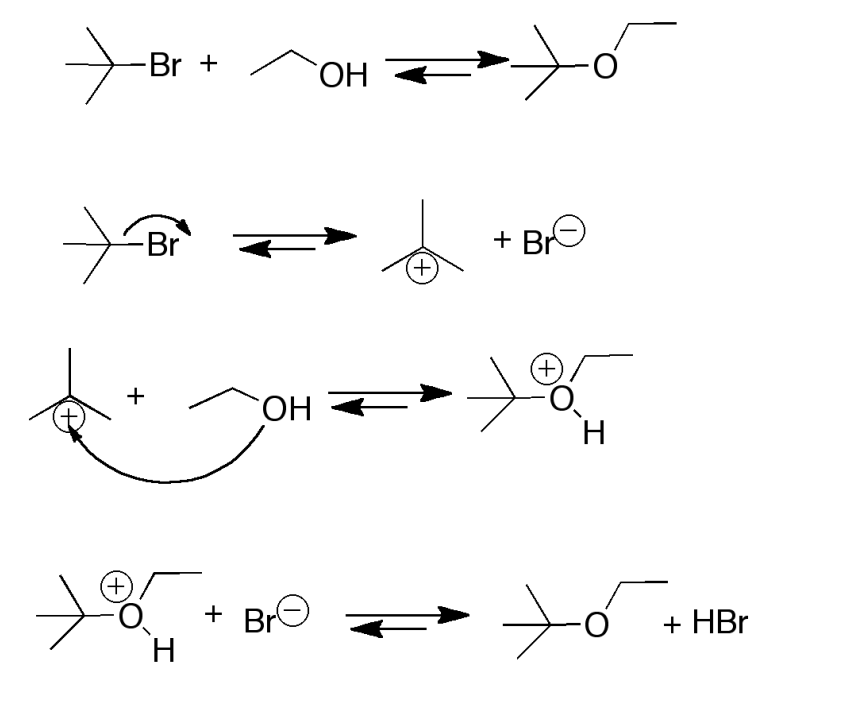
Provide the mechanism for reaction of ethanol with bromotriphenylmethane
SN1 reaction so first Br reversibly dissociates in alkyl halide
Then the oxygen from the alcohol attacks the positively charged alkyl group
The bromine then deprotonates the OH group to form an ether and HBr
How are you going to determine whether the reaction is completed?
pH using litmus paper, if acidic that means HBr is still present meaning the reaction is still going
Why do you not observe E1 product in reaction of ethanol with bromotriphenylmethane?
This is because there are no sp3 carbons with protons next to the halide group
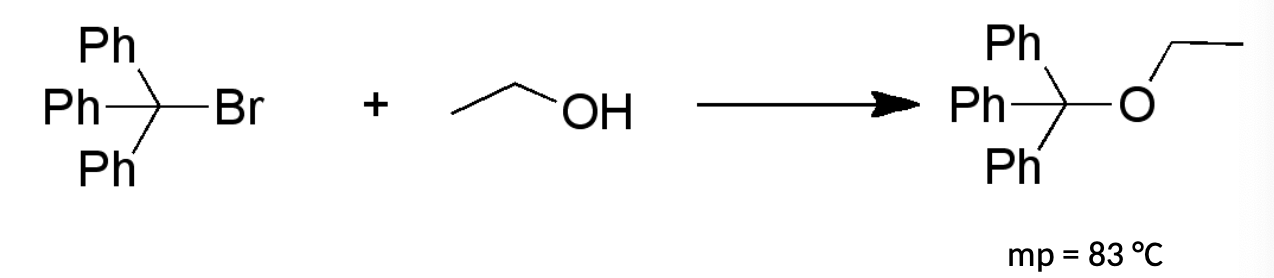
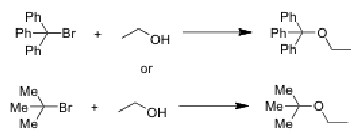
Which reaction should be faster and why?
Top one because the carbocation intermediate from the SN1 reaction is stabilized from the pi bonds that come from the benzene rings

Propose how to synthesize:
Top one, primary alkyl halide
Bottom one, benzylic alkyl halide
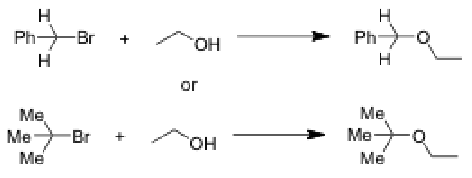
In which of the following reactions you should observe a higher amount of the product of E1 elimination? Why? Give the structure of E1 product in both cases.
More E1 in bottom reaction because it has beta protons (from methyl reaction)
Top reaction doesn’t work because it doesn’t have an sp3 protons to form double bond