Amino Acids
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
contrast transcription and translation
transcription forms RNA from DNA, both use nucleic acids to form but they slightly differ;
translation forms proteins from RNA, RNA uses nucleic acids protins use amino acids as the monomer
In eukaryotes, what polymer goes through replication:
A) RNA
B) Protein
C) DNA
D) polysaccharides
C) DNA
how can transcription be aliken to copying instructions in your own words?
DNA and RNA both use nucleic acids, RNA uses ribonucleic acids while DNA uses deoxyribonucleic acids
How did Rosalind Franklin’s work on X-ray diffractions of DNA contribute to Crick and Watson’s discovery?
Watson and Crick discovered how the structure of DNA looks, Franklins work confirmed the helical structure of DNA
Contrast reverse transcription and transcription
Reverse transcription from DNA (cDNA) from RNA using reverse transcriptase
Transcription forms RNA from DNA using RNA polymerase
Reverse transcription makes DNA from RNA so it can be integrated into the host genome in:
A) Retrovirus (HIV)
B) ssRNA (+) virus (poliovirus)
C) dsRNA virus (reovirus)
D) ssRNA (-) virus (influenza)
A) retrovirus (HIV)
How do RNA viruses differ from the central dogma?
They start with an RNA genome, no DNA, so their genome can be translated directly to form protein or the complementary strand is formed from the genome to be translated into protein
RNA Virus: I) SARS II) influenza III) HIV
A) I
B) II
C) I and III
D) I and II
D) I and II
How does ncRNA (non-coding RNA) deviate from the central dogma?
NcRNA does not get translated like mRNA, but instead can perform functions on its own without further modifications
NcRNA: I) rRNA II) mRNA III) tRNA
A) I, II and III
B) I and III
C) II and III
D) I and II
B) I and III
How does translation incorporate the use of ncRNA?
tRNA brings the amino acids corresponding to the mRNA codons. rRNA forms the complex where translation occurs
How did epigenetics change the way we saw simple genetics?
Simple genetics saw changes in phenotype due to changes in the genotype, but epigenetic’s proved that even with no changes in the DNA sequence itself, modifications to the DNA can result in different phenotypes
How does epigenetic’s explain how muscle cells are so different from skin cells?
The DNA sequence is the same in both cells, however epigenetic mechanisms (histone modification, methylation) modified their expressions resulting in only certain genes being transcribed
What are the 6 functions of proteins?
Catalyst, structure, mobility, transport, immunity, communication
Why are amino acids termed alpha amino acids
They contain an alpha carbon (carbon attached to carbonyl group), which is attached to an alpha hydrogen, R group and amino group, also making it a chiral carbon
Why is glycine the only non chiral amino acid?
Its side chain is an H, a carbon is chiral if it is attached to 4 different groups, but the alpha carbon of glycine would be attached to 2 H’s
Why is only cysteine in the R configuration?
The sulfur in the side chain has a higher atomic number than the oxygen in the carboxylic acid group. The priority of the groups would go in the clockwise direction
Why is the zwitterion form the dipolar form?
the amino group is protonated forming a positive charge and carboxylic acid group is deprotonated forming a negative charge
what promotes the zwitterion form?
amino acids in neutral pH (4-7)
what promotes the positively-charged amino acid species?
pH of 1 because there are more protons and the carboxylic acid group will be protonated
what promotes the negatively-charged amino acid species?
pH of 9 because the [H+] has decreased and the amino group will be deprotonated
what is the reaction that forms peptide bonds?
nucleophilic addition elimination reaction
what attacks the carbonyl carbon in order to form the peptide bond?
the electron pair on the nitrogen from the amino group
the other name for a peptide bond?
amide bond
how is the amide bond resonance stabilized?
the nitrogens electrons can be delocalized to the carbonyl oxygen forming a double bond
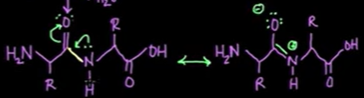
why the polypeptide chain is considered the backbone?
peptide bonds are very rigid and planar with very little rotation about the bond
is the entire polypeptide chain a rigid structure?
no, there is still free rotation about the alpha carbon
what is a residue in a polypeptide chain?
each amino acid in the polypeptide chain after being linked
why is acid hydrolysis considered non specific way of cleaving peptide bonds?
if you toss a polypeptide chain into a pot with strong acid and add some heat, all of the peptide bonds would be cleaved leaving a bunch of amino acids
why is proteolysis considered specific way of cleaving peptide bonds?
enzymes called proteases will only cut peptide bonds between certain specific amino acids, not all of them, resulting in fragments of peptides
why does histidine work well to be used in the active sites of our enzymes?
its pKa is around 6.5 which is close to physiological pH, so it will be in dipolar/zwitterion form
why does proline have a secondary alpha amino group?
its side chain forms a cyclic structure with the amino group, covalently bonded to the alpha nitrogen
how does the H side chain of glycine make this amino acid unique?
it makes the alpha carbon not chiral, and because the hydrogen is a small atom for a side chain, allowing free rotation
why are proline and glycine considered alpha helix breakers?
proline’s secondary alpha amino group introduces kinks into the helix, glycine’s ability to freely rotate also does this, disrupting the alpha helix structure
what is a thiol group?
SH
why do disulfide bridges form in oxidized environments?
the thiol group (SH) exists in a reduced environment, when put into an oxidized environment the H is lost and the sulfurs can bond
how does the intracellular environment stay as a reducing environmnet?
antioxidants in the cell prevent oxidation, thus preventing disulfide bridge formation
difference between cysteine and cystine
cysteine is the reduced form (e like you have electrons) and cystine is the oxidized form (loss of e like loss of electrons)
how does the fisher projection of a L amino acid differ from a D amino acid?
the amino group of the L amino acid is on the left side while for the D configuration it is on the right side
how are L and D form amino acids related? which are found in humans?
they are enantiomers of each other. L form
what is the isoelectric point (pI)?
the pH at which the amino acid exists in its zwitterion form, it is neutral
how is the isoelectric point found?
taking the average of the pKas surrounding the zwitterion form of the amino acid (ie. zwitterion is between amino group pKa and R group pKa)
which amino acids are non-polar with alkyl side chain?
glycine (gly, G), valine (val, V), isoleucine (ile, I), alanine (ala, A), leucine (leu, L), methionine (met, M), proline (pro, P)
which amino acids are non polar with aromatic side chains?
phenylalanine (phe, F) and tryptophan (trp, W)
which amino acids are polar with neutral side chains?
serine (ser, S), threonine (thr, T), asparagine (asn, N), glutamine (gln, Q), cysteine (cys, C) tyrosine (tyr, Y)
what do S, T, N, Q, C and Y have in common as polar neutral AAs?
their side chains contain O or S, which are e- withdrawing, creating localized negative charge around it and the rest of atom has localized positive charge
which amino acids are polar with acidic side chains?
aspartic acid/aspartate (asp, D) and glutamic acid/glutamate (glu, E)
what do D and E have in common as polar acidic amino acids?
they have COOH group in their side chain, a strong H donor
which amino acids are polar with basic side chains?
histidine (his, H), lysine (lys, K), arginine (arg, R)
what do K, R and H have in common as polar basic AAs?
they have N’s in their side chain, proton acceptor