KBKF15 - WEEK 4 - METABOLSIM - GLYCOLYSIS
1/31
Earn XP
Description and Tags
PLUGGA MEST PÅ ÖVNINGARNA (?)
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
32 Terms
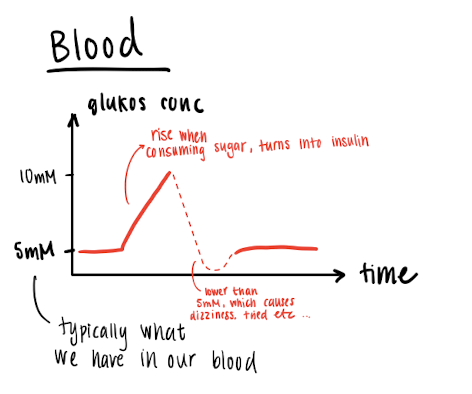
Glucose level in the blood
Normally, we have about 4,5g in our blod, which supplies for blood cells, brain etc.
Glucose is stored as glycogen (polymer of glucose) with we normally have 1 kg of. 1 kg glycogen binds to 4 kg water, which is what we usually lose.
BMI : Body ,ass index, how much fat we have, too much glycogen converts into fat.
We wanna consume sugars with low BI.index, so we don’t get a glucose dip.
Metabolism is divided into 2 parts
Catabolism : Breaks down the food, and creating energy from it.
Anabolism : It uses the created energy to build up compounds.
Metabolic syndrome
Collapse of metabolism ( high levels of sugar, fat) which leads to diabetes and obesity etc
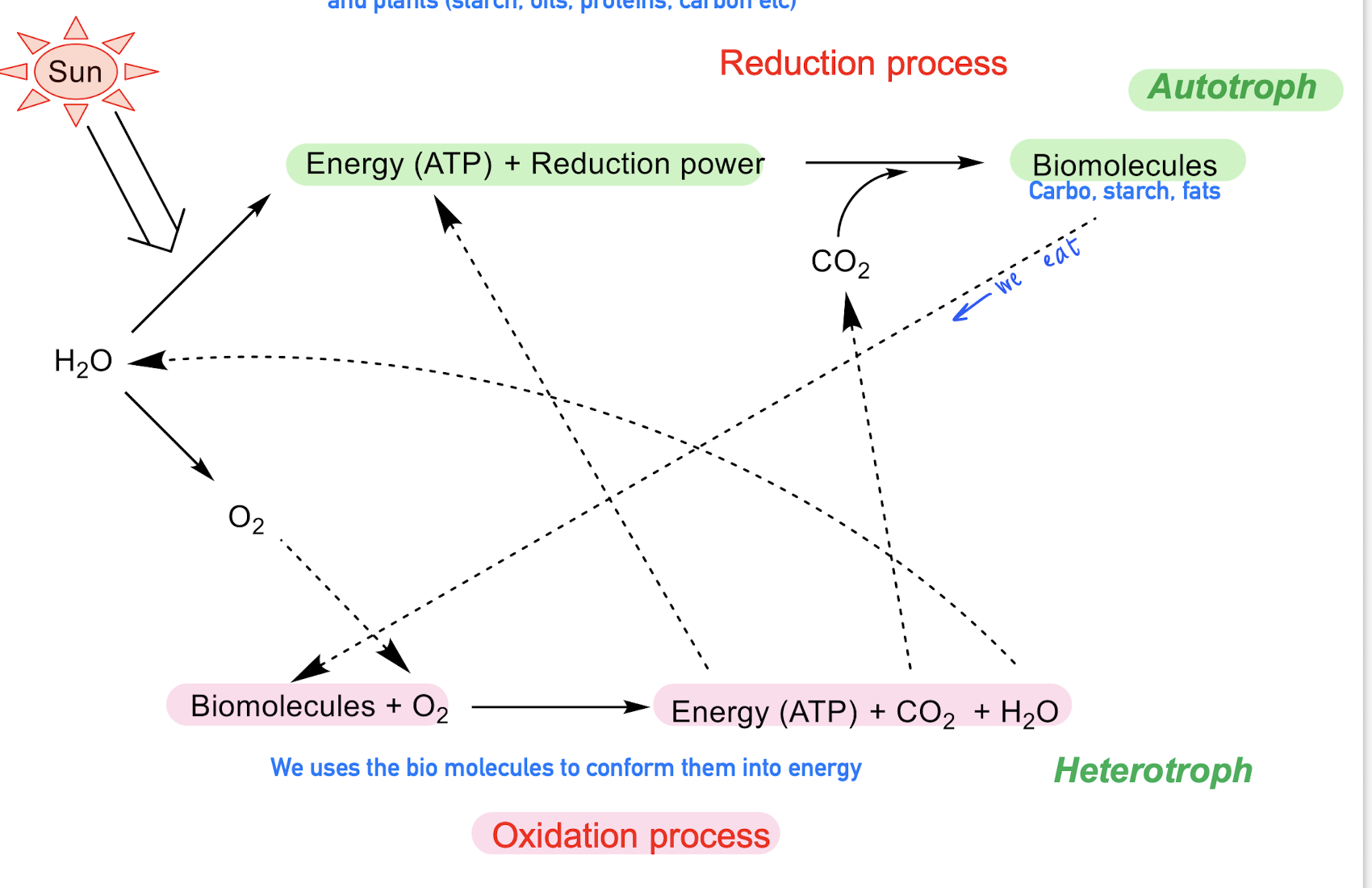
Energy source for sustaining life
We are very dependent of the sun in 2 different way, Reduction process and Oxidation process.
For Autotrophs, water, together with ATP, Co2 and reduction power —> it generates biomolecules (carbs, lipids)
For Heterotrofs, They consumes the biomolecules, and together with oxygen they oxidaize into Energy (ATP), Co2 AND H20.
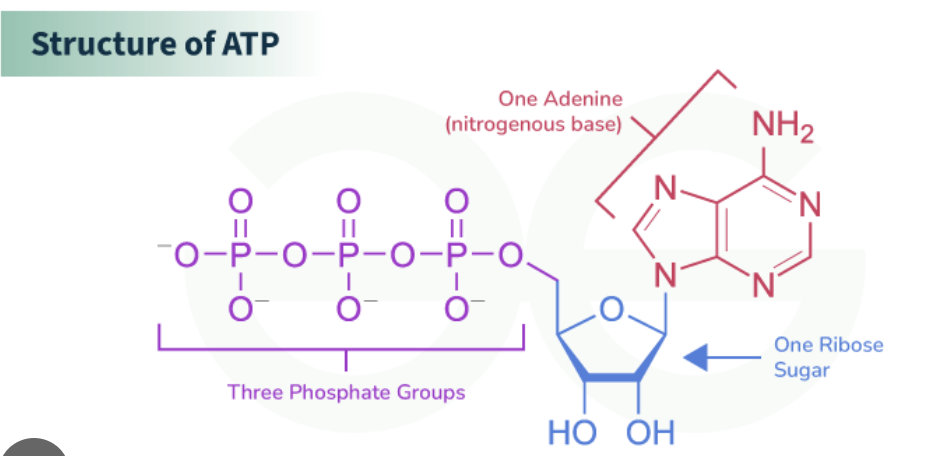
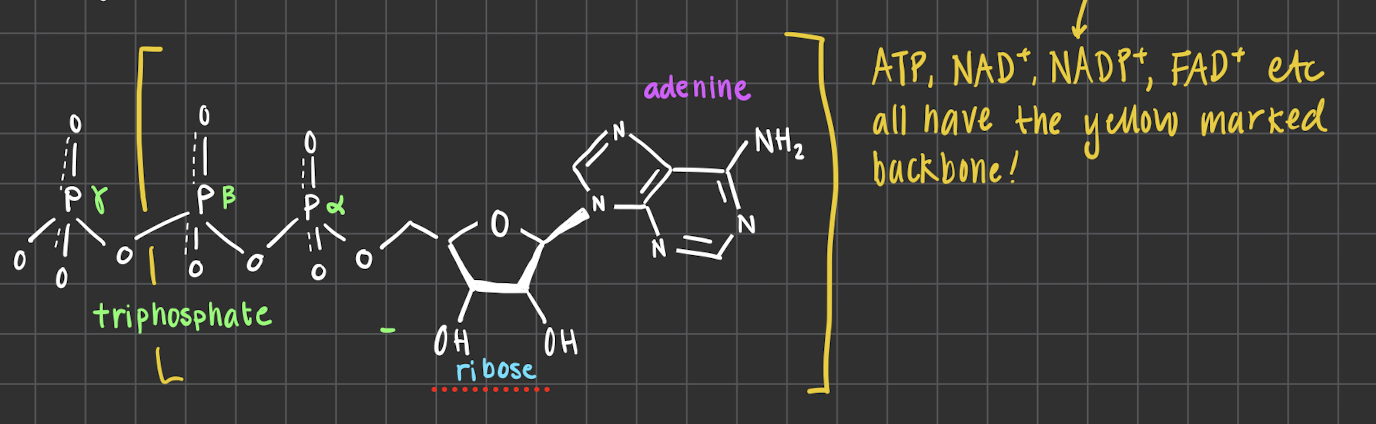
ATP structure and energy level
Contsructed by a Ribose sugar Adenine and Triphosphate.
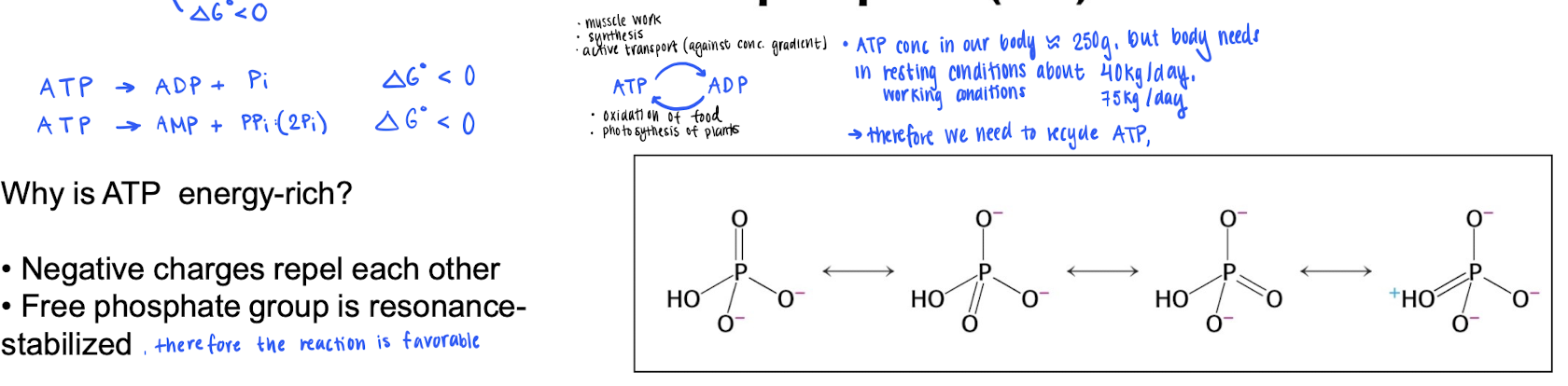
The large amount of energy is stored in the phosphates, they are (-) charged to prevent them from dissosiating from the molecule. However, dissasiation is very favorable (ΔG < 0) : free phosphate is resonance stabilized.
Something to think about is the fact that we only have around 250g of ATP, but the body needs 40-75 kg / day. Therefore, we need to recycle !
Gemensamt structure for energy carriers
almos all of the energy rich carriers has a base structure, this includes ATP, NADH, NADPH, FAD …
Ribose sugar : for stability of the molecule, binds the phosphate and adenine group together, also found in our DNA
Adenine : allows the participation in formation of nucleic acids, involved in reactions that gives energy to the cell
Di-Phosphate : (-) charged to hold the molecule together, holds large amount of energy.
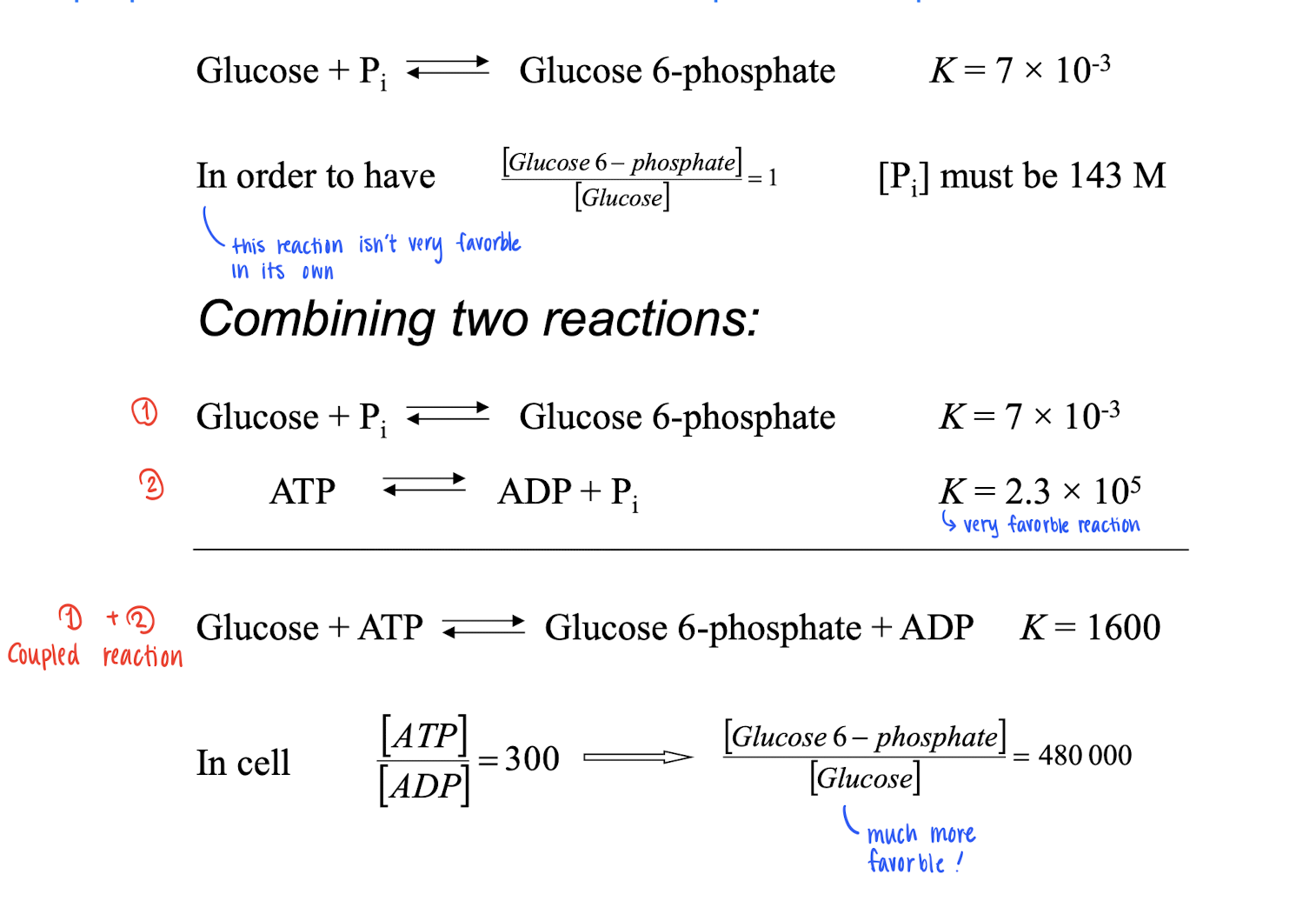
Coupled reaction
For a reaction to be favorable, (ΔG < 0), and some times, that doesn’t accour. However, you add more step reactions to make the TOTAL (ΔG < 0). So you can mix reactions to make it more favorable!
Example of energy carriers
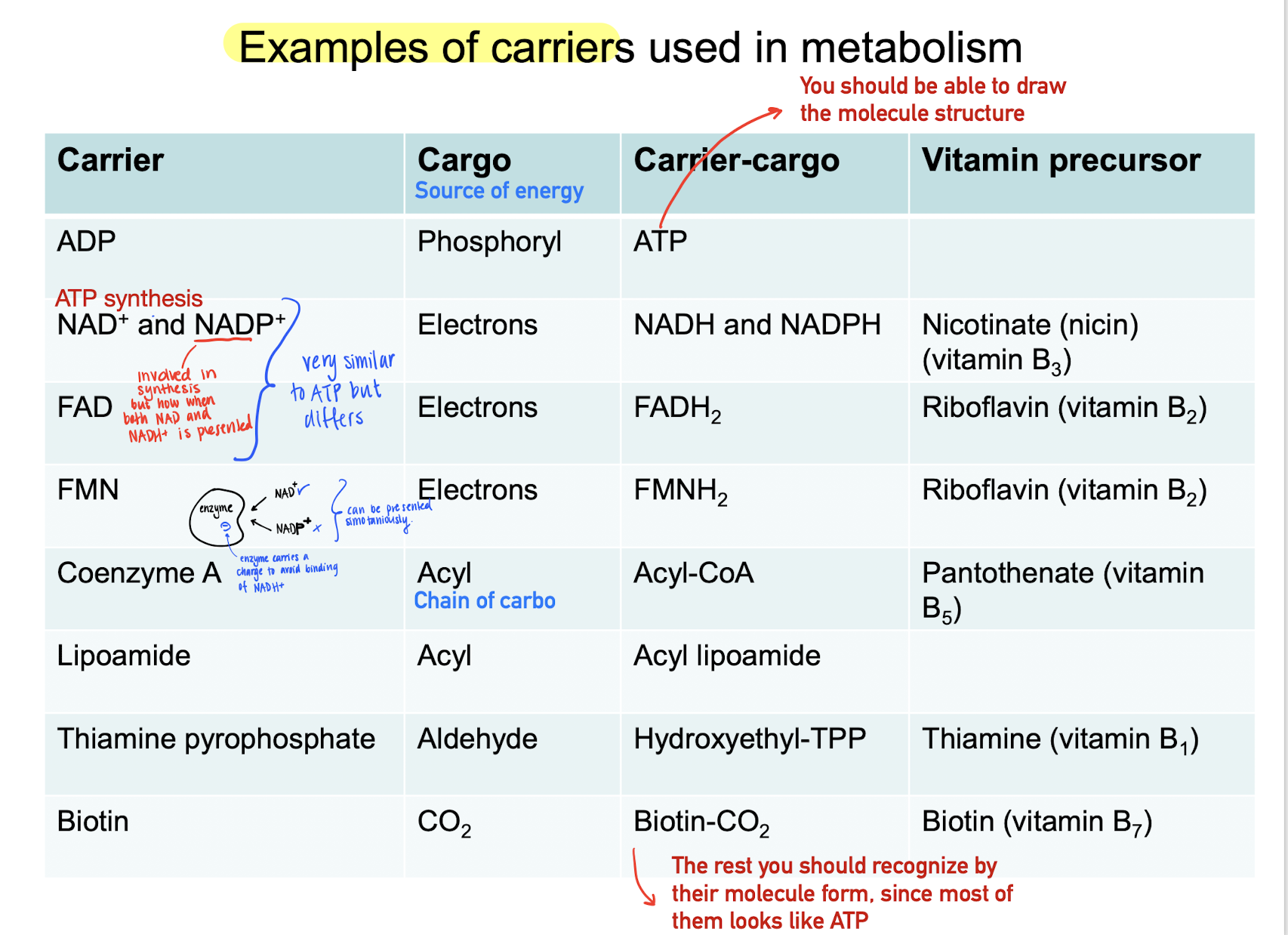
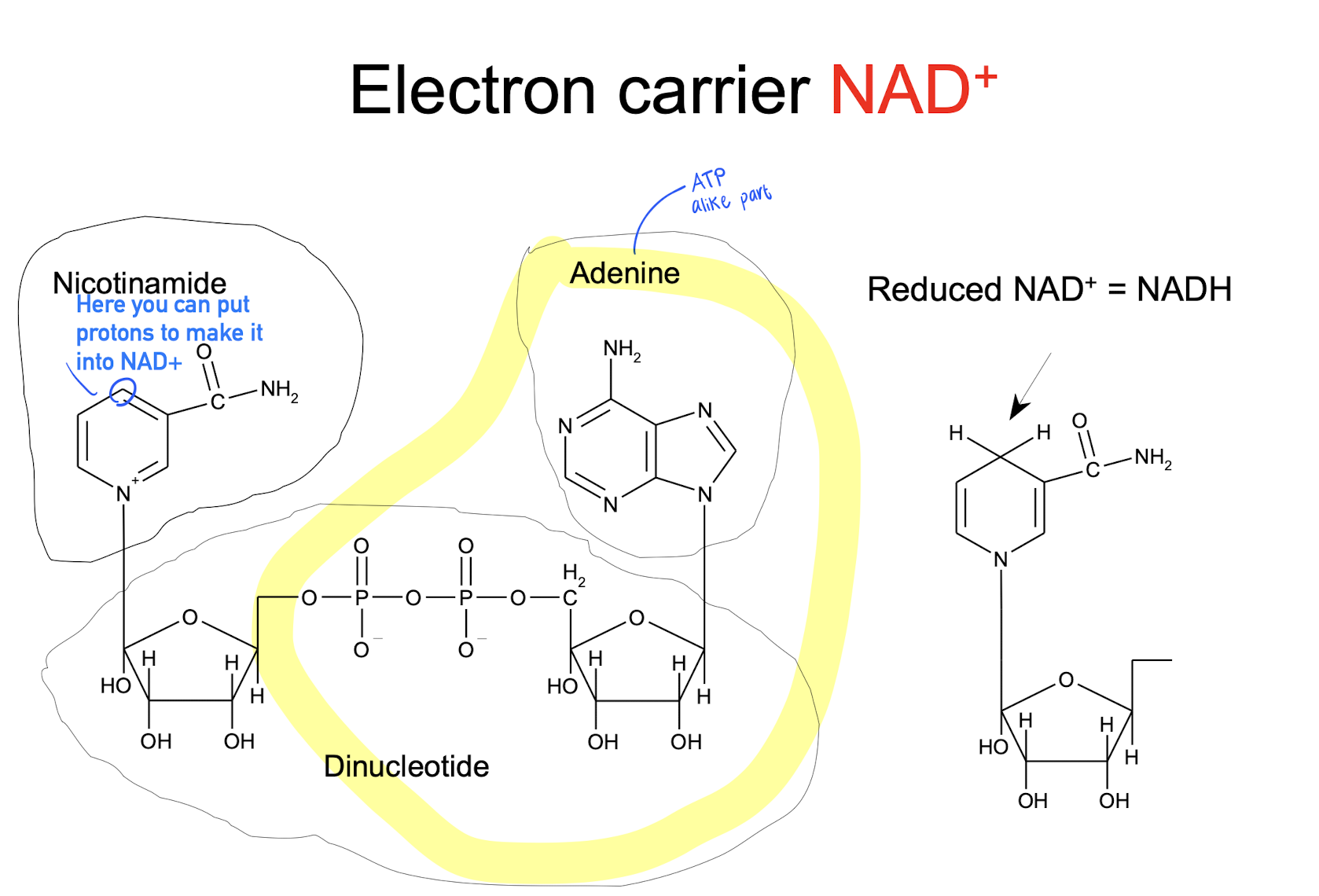
NAD+, NADH (reduced form)
Electron carrier
Used in glycolysis (?)
Used in ATP synthesis
NADP+, NADPH (reduced)
Involved in biosynthesis as a reducing agent.
The phosphate group in the ribose tells NADH and NADPH apart.
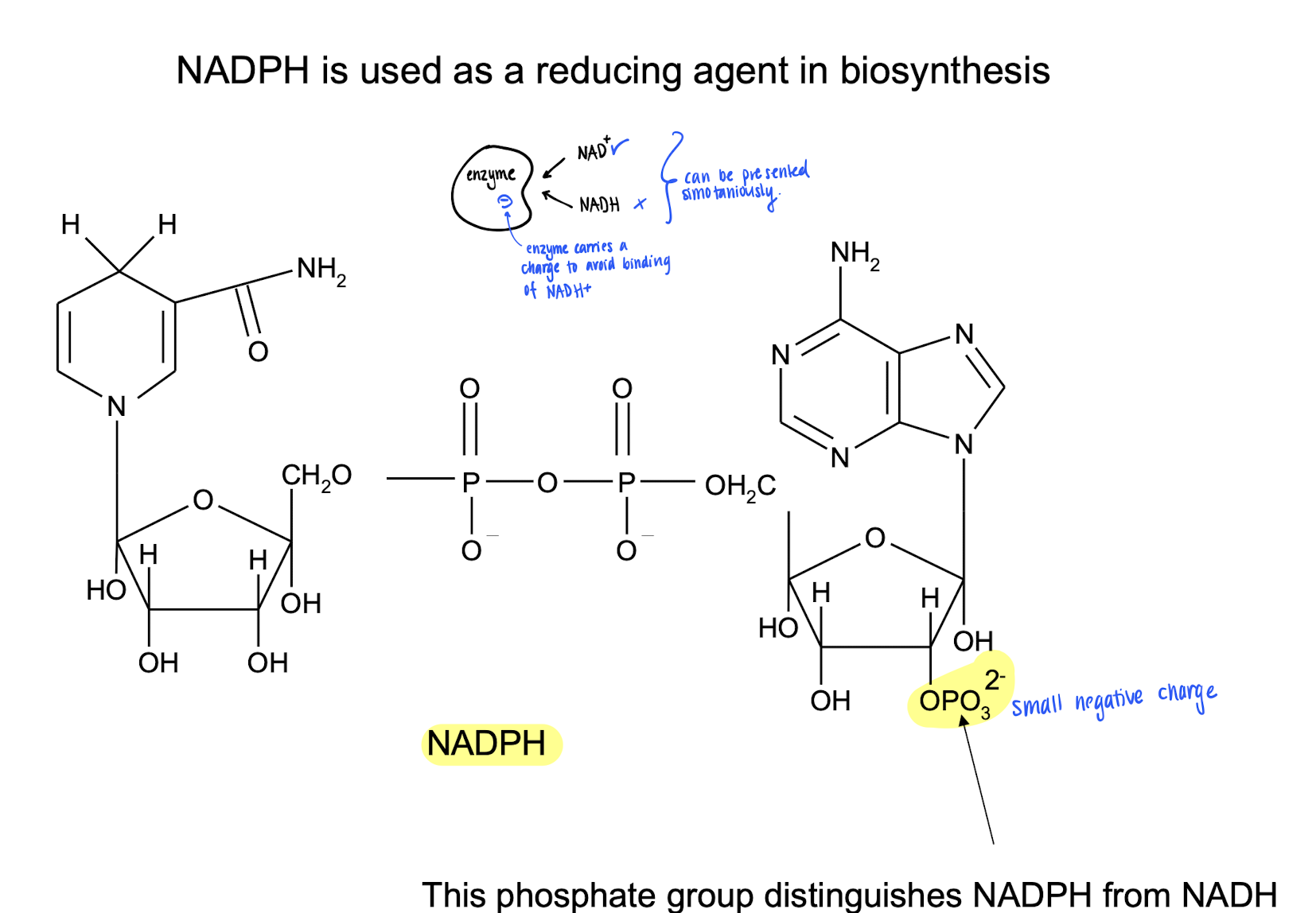
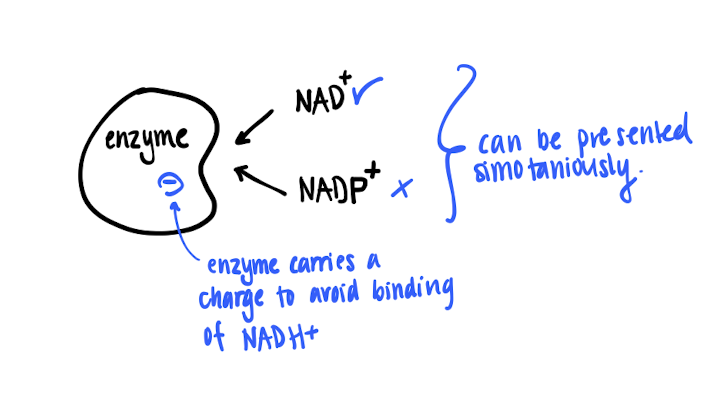
How does enzymes tell NAD+ and NADP+ apart?
They are very similar, but involved in completely different reactions. But they can be presented simultaneously
ATP-forming enzymes carries a negative charge, then only NAD+ is able to bind on the active site.
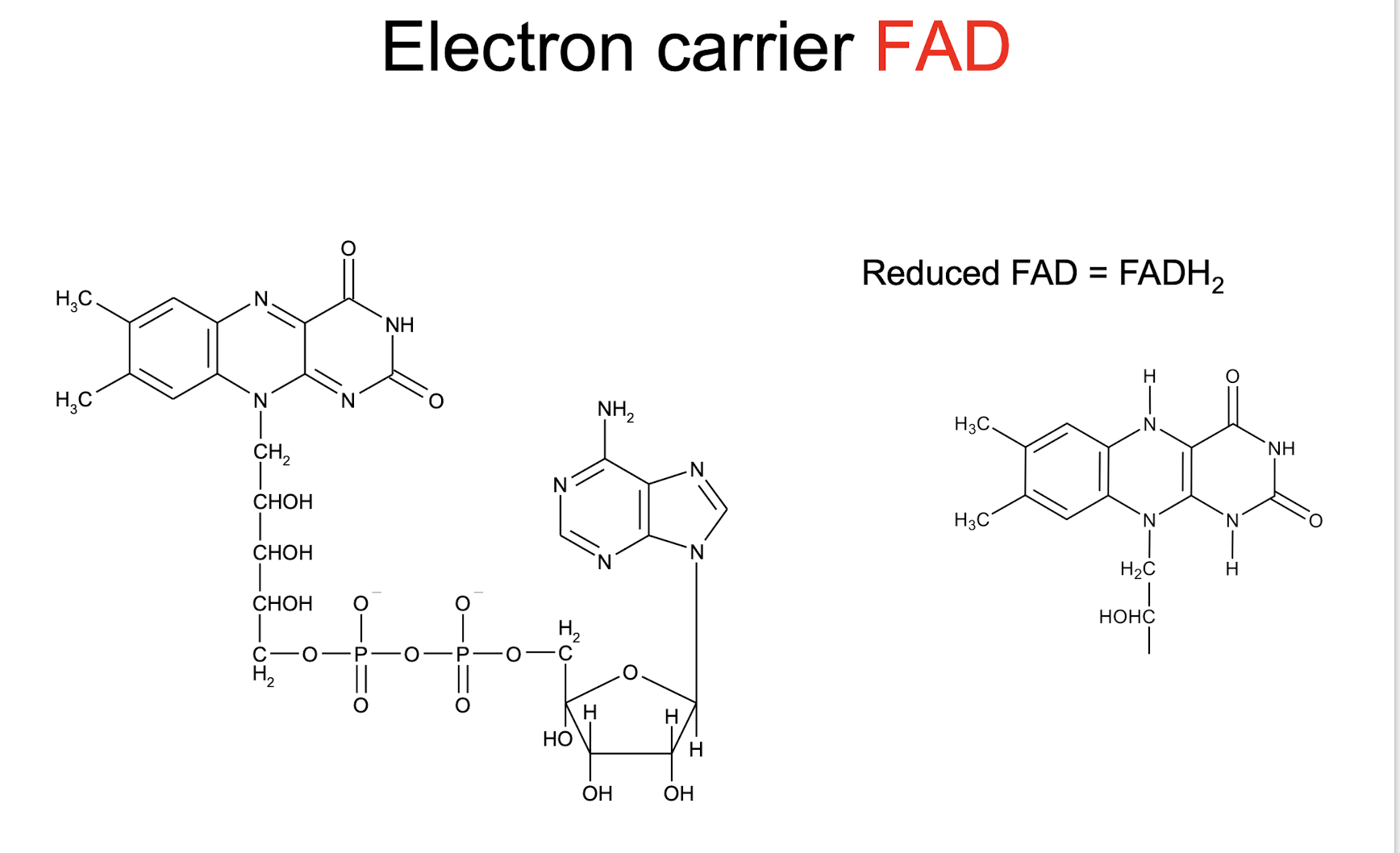
FAD, FADH2 (reduced)
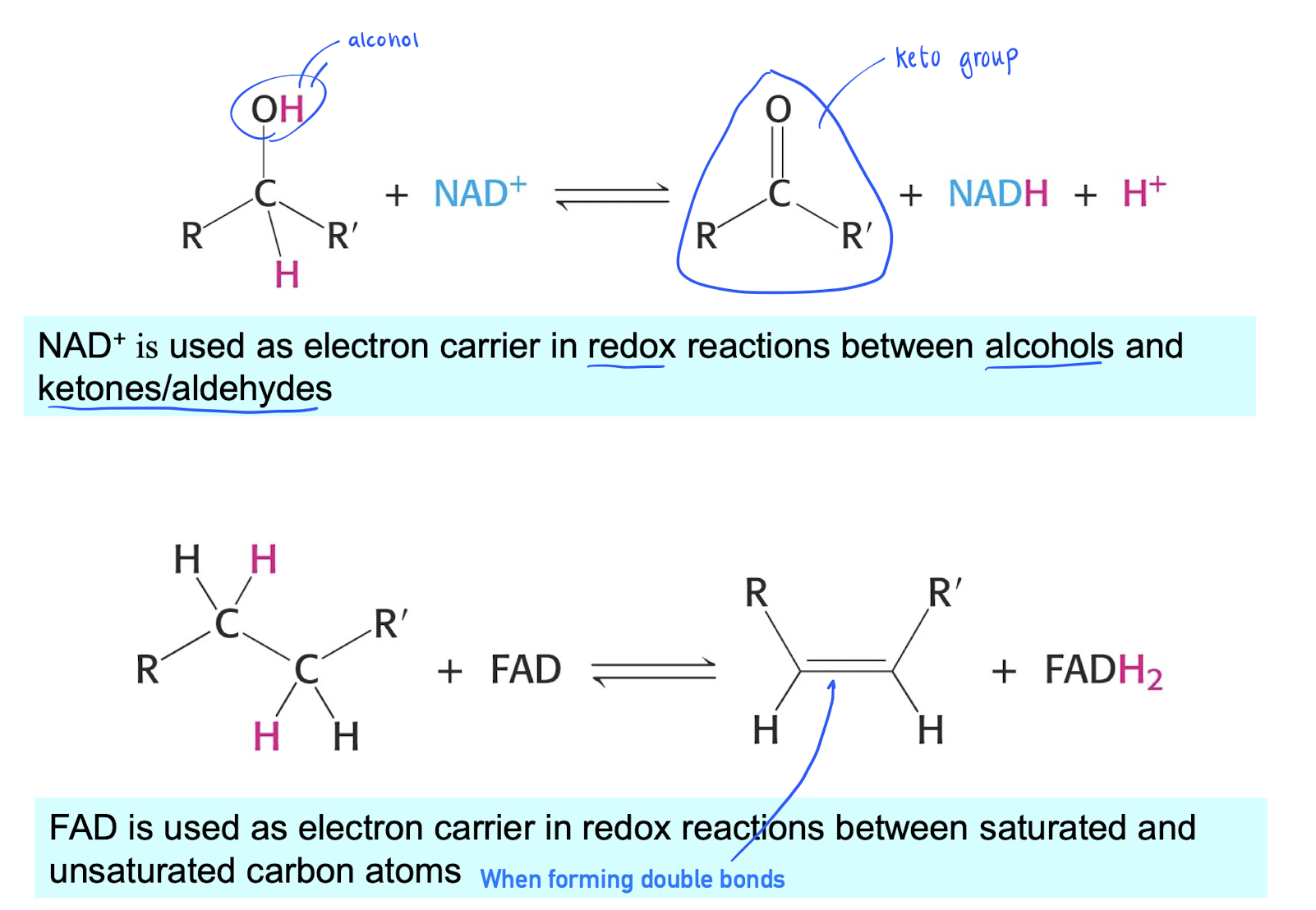
Difference in reactions (NAD+ & FAD)
NAD+ : e- carrier in redox between alcohols and keton groups.
FAD : e- carrier in redox between saturated / unsaturated carbon atoms (double bonds)
CoA
Carrier for short and long acyl-groups (chain of carbohydrates.
Carries much energy
Kinetically stable : slow at reaching low energy due to high (EA), which prevents immidiate transformation.
Thermodynamically labile : molecule not at lowest energy, has tendency to convert into more stable form (ΔG < 0)
therefor release a lot of energy (?)
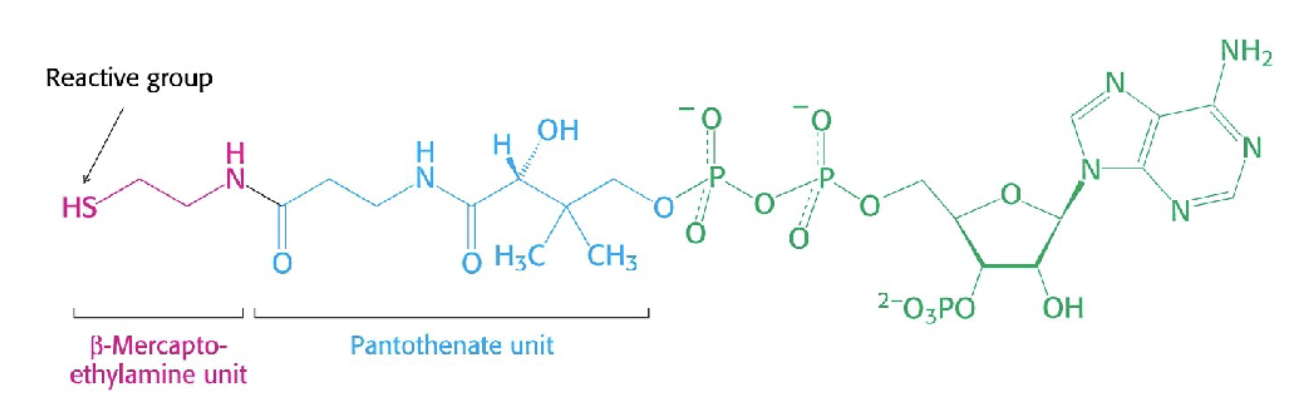
Why is creatine good?
Ingesting creatine —> higher concentration creatine —> increased level of creatine phosphate, this is used to phosphyrolize ADP back to ATP for energy generation.

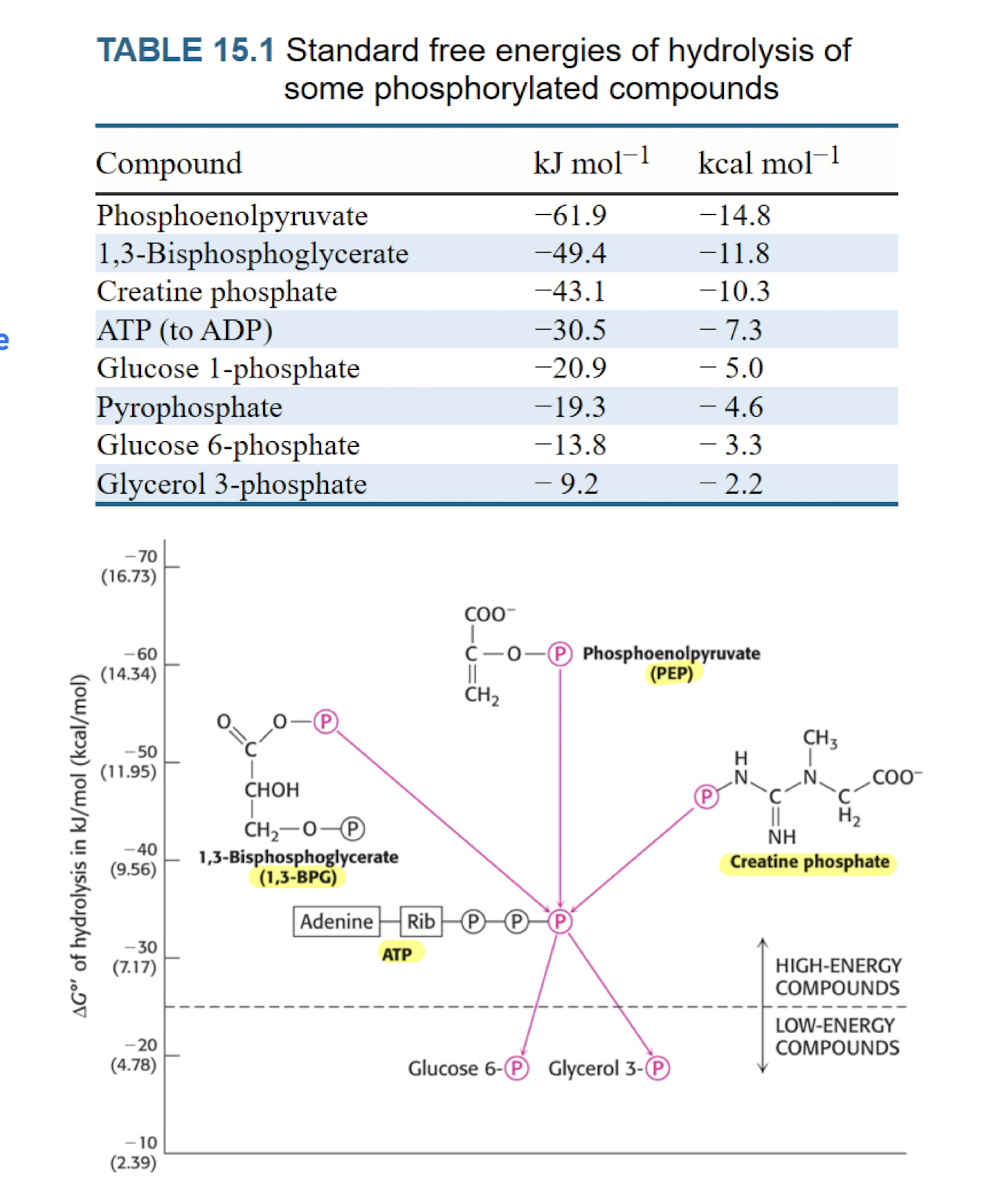
Molecules that can donate phosphate to ADP
SLP : Substrate-level Phosphorylation
Donation to make ADP into ATP
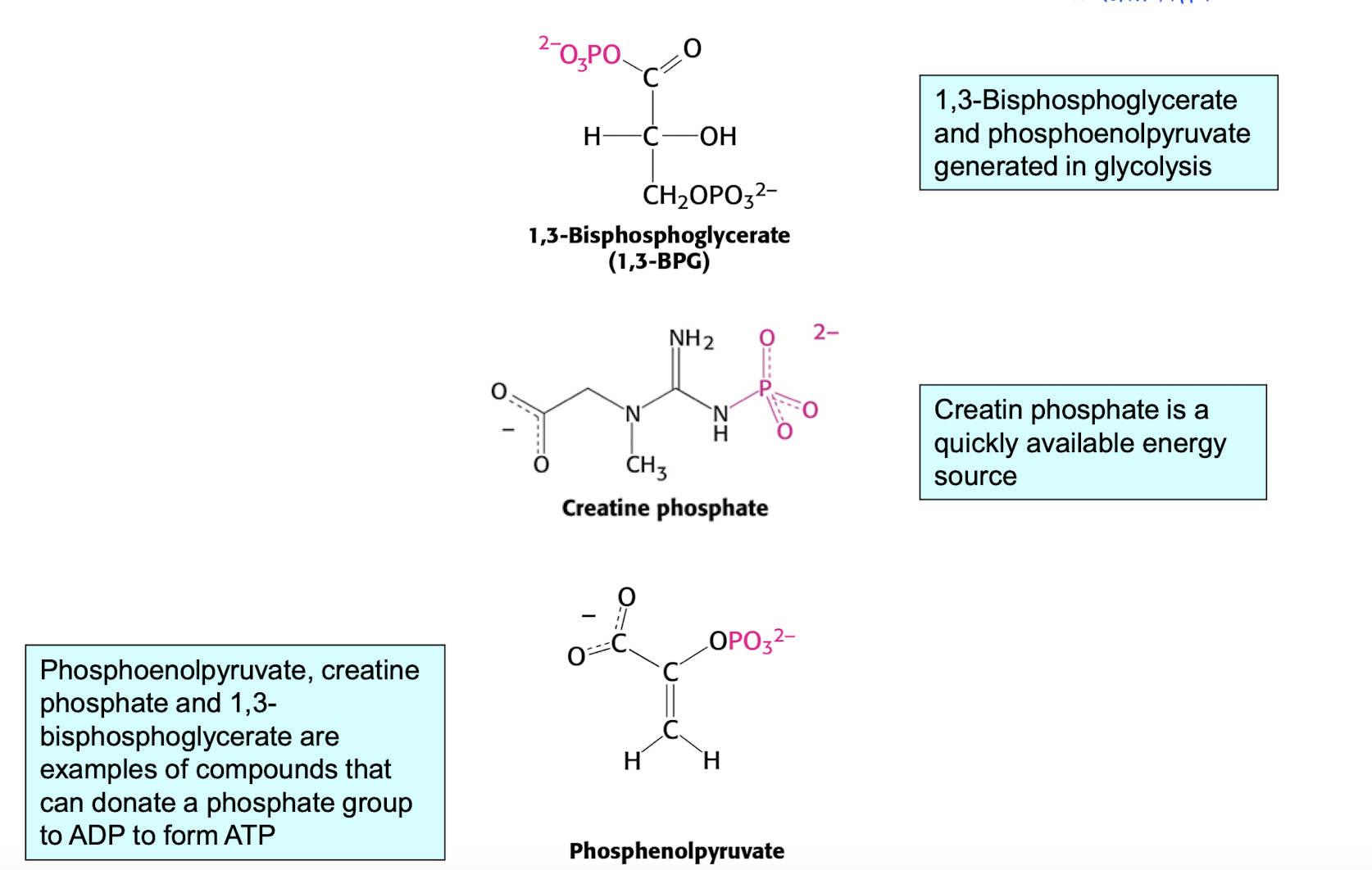
Oxidative phosphorylation - more detailed in week 5
A part of the electron transport chain?
This seems to be a very simplified version (?)
Creation of H+ gradient, H+ is pumped outside the mitrocondria matrix. The inside of the mitocondria is now more (-).
- NADH —> (HAD+) + (H+)
- FADH2 —> (FAD) +(2 x H+)ATP-syntase, the H+ gradient is used to phosphyorate ADP into ATP. Energy is created through H+ going through ATP-syntase.
Food —> Energy (good to know the picture)
So many steps because we don’t wanna generate heat, but we wanna generate energy !

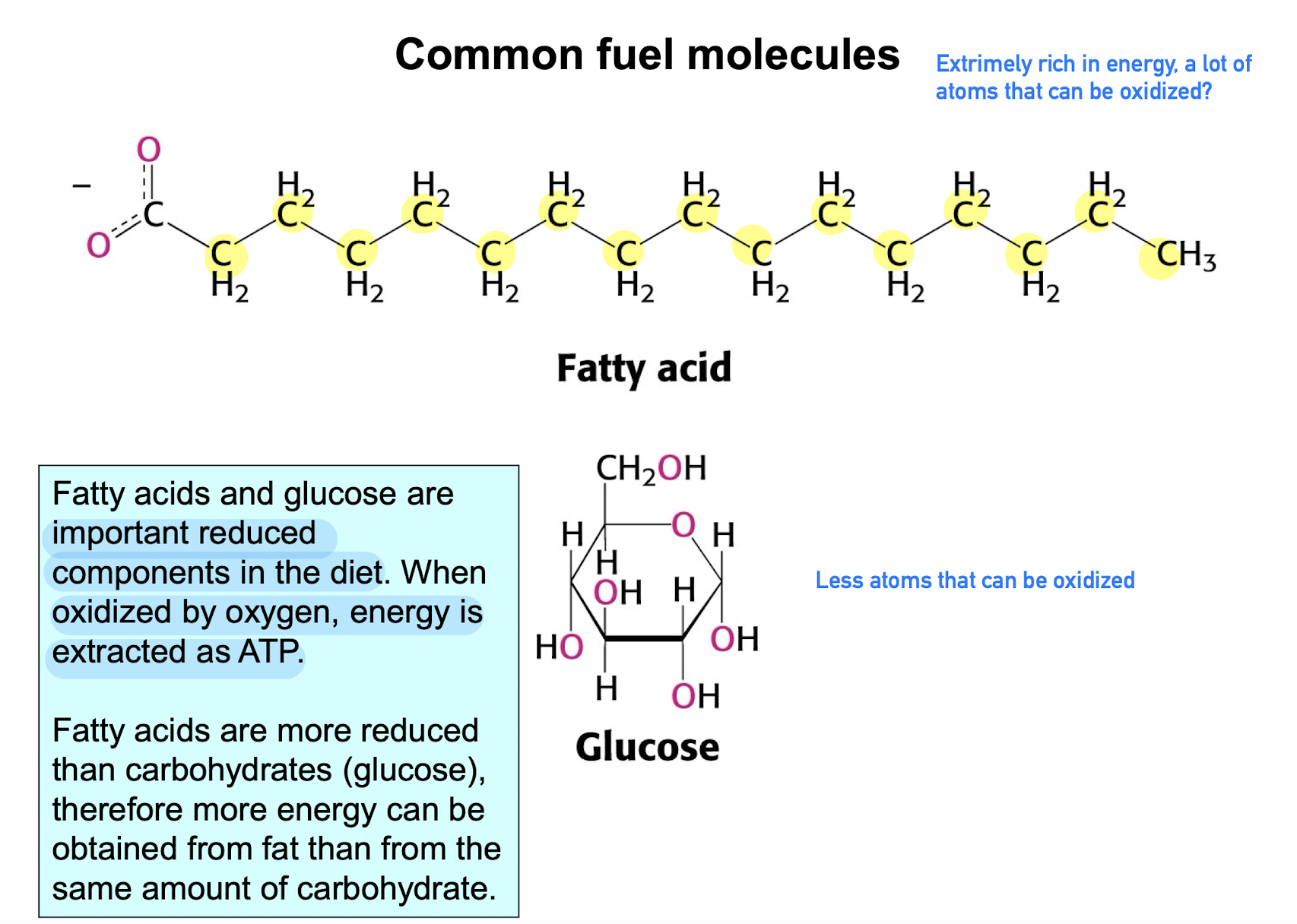
Common fuel molecules (Reduced molecules)
important reduced molecules in diet that release energy (extracted as ATP) when oxidized.
Fatty acids and glucose are common
Fatty acids are more reduced than glucose (less O i guess), therefore, it generates more energy when oxidized.
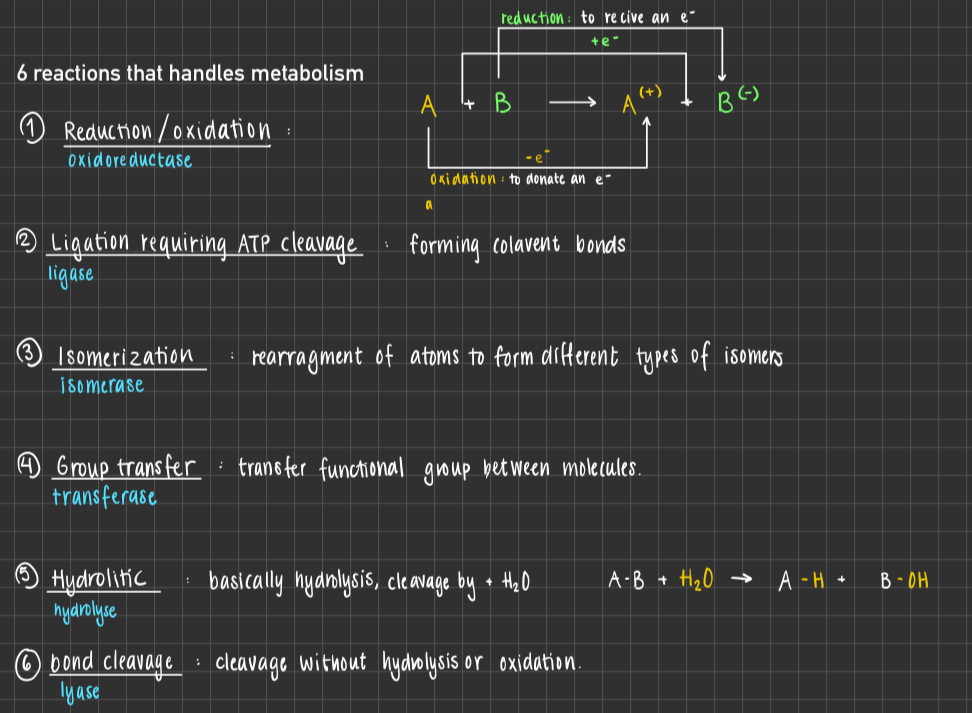
6 reactions what handles metabolism
Oxidation reduction : Electron transfer using Oxidoreductase
Ligation reguiring ATP cleavage : Formation of covalent bonds (t.ex C-C) using Ligase
Isomerization : Rearrangment of atoms to form isomers using isomerase
Group transfer : Transfer of a function group from 1 molecule to another using transferase
Hydrolitic : Cleavage of bonds b the addition of water, instead pf (A-B + H2O —> A-H + B-OH) using hyrdrolyase
Bond cleavage : substrate splits into 2 products without hydrolysis or oxidation using lyase
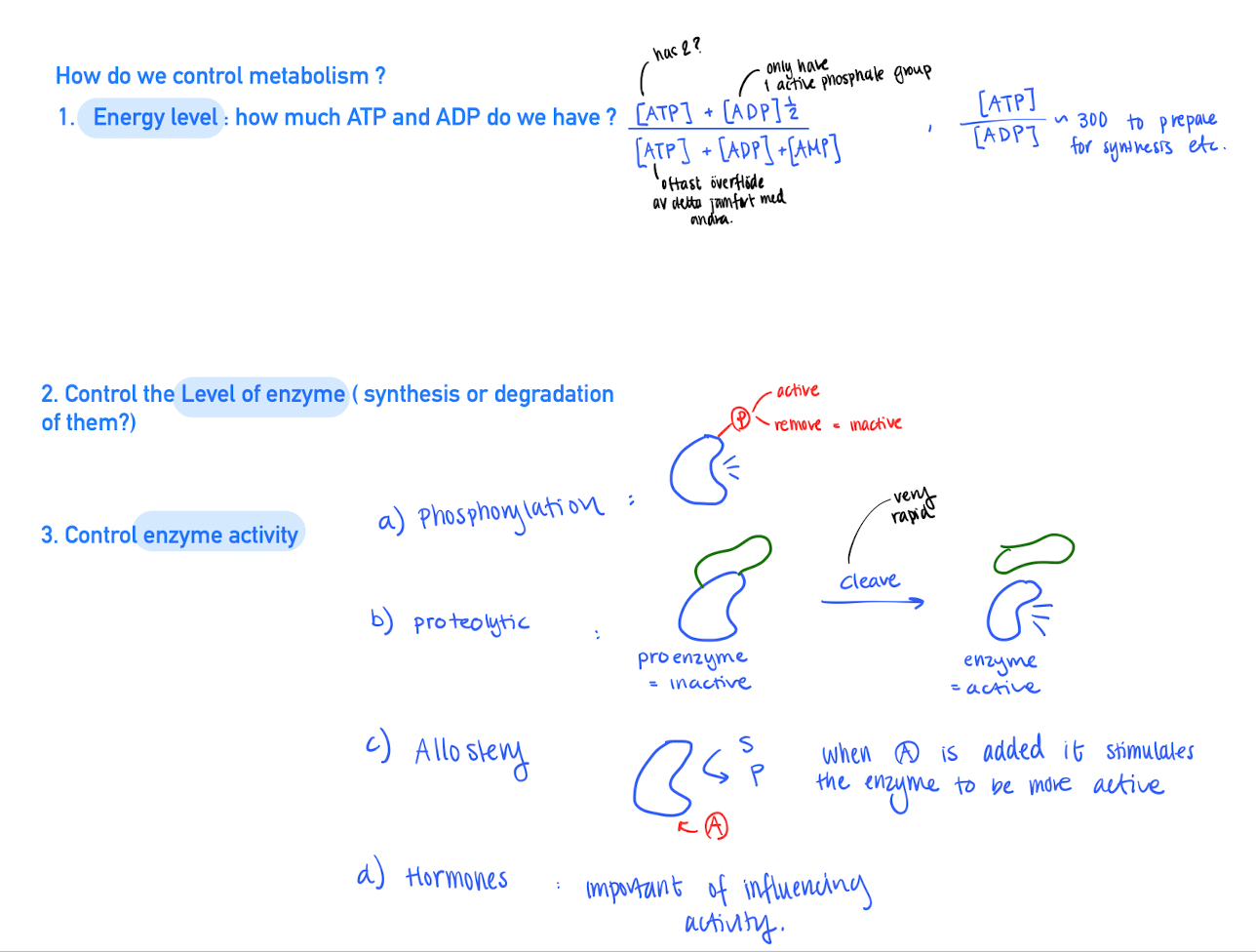
Ways to control metabolism
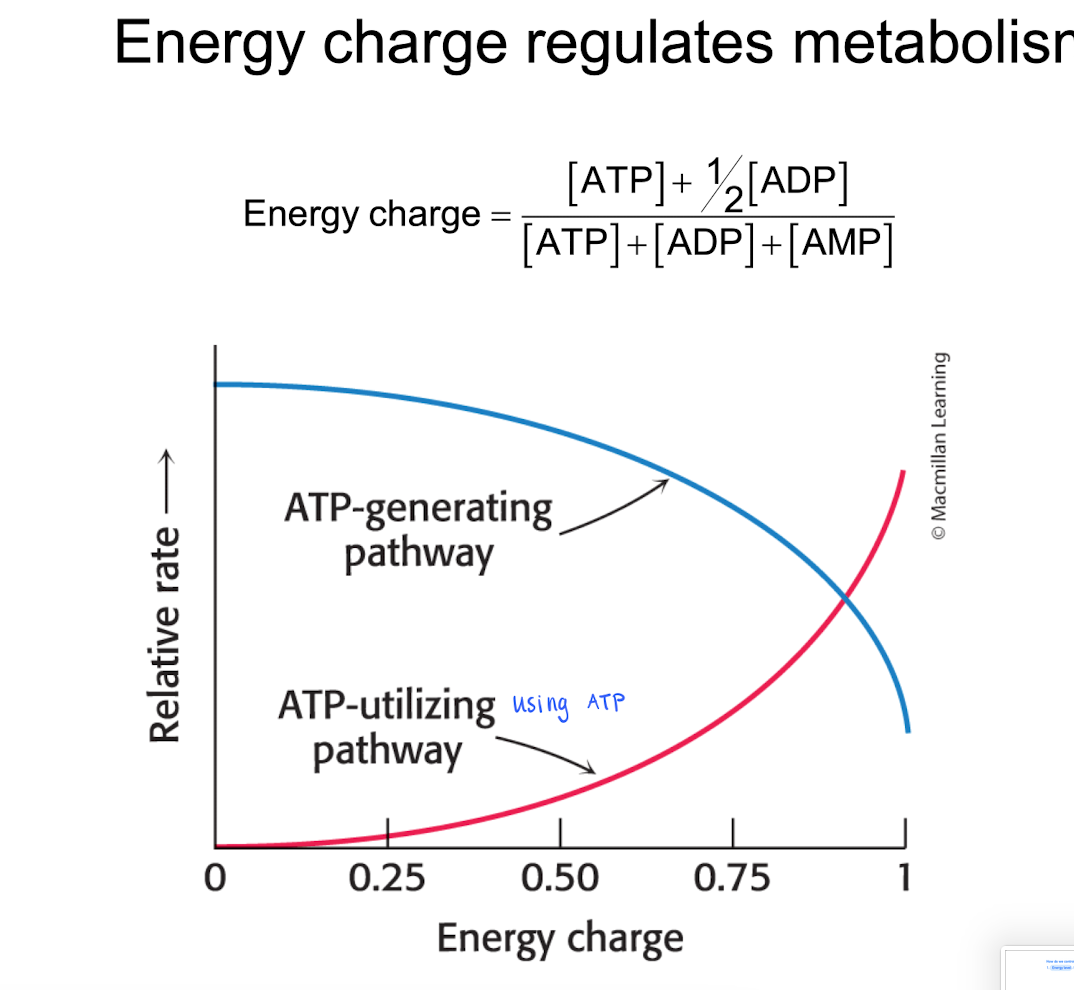
Energy level : Hoe much ATP do we have? The energy charge is based on the concentration of A(T,A,M)P
Control Enzyme activity
- Phosphorylation : some enzyme needs a phosphate to activate.
- Proteolytic : Enzyme that needs to be cleaved to be activated
- Allosteric : When a molecule is bond to the enzyme, the activity increaes / decreases.
- Hormones : important first messanger that influence the enzyme actvities.
Different metabolism steps adressed in this course
Glycolysis, Gluconeogenesis (glycolysis but the other way around) Citric acid cycle, Oxidative phosphorylation
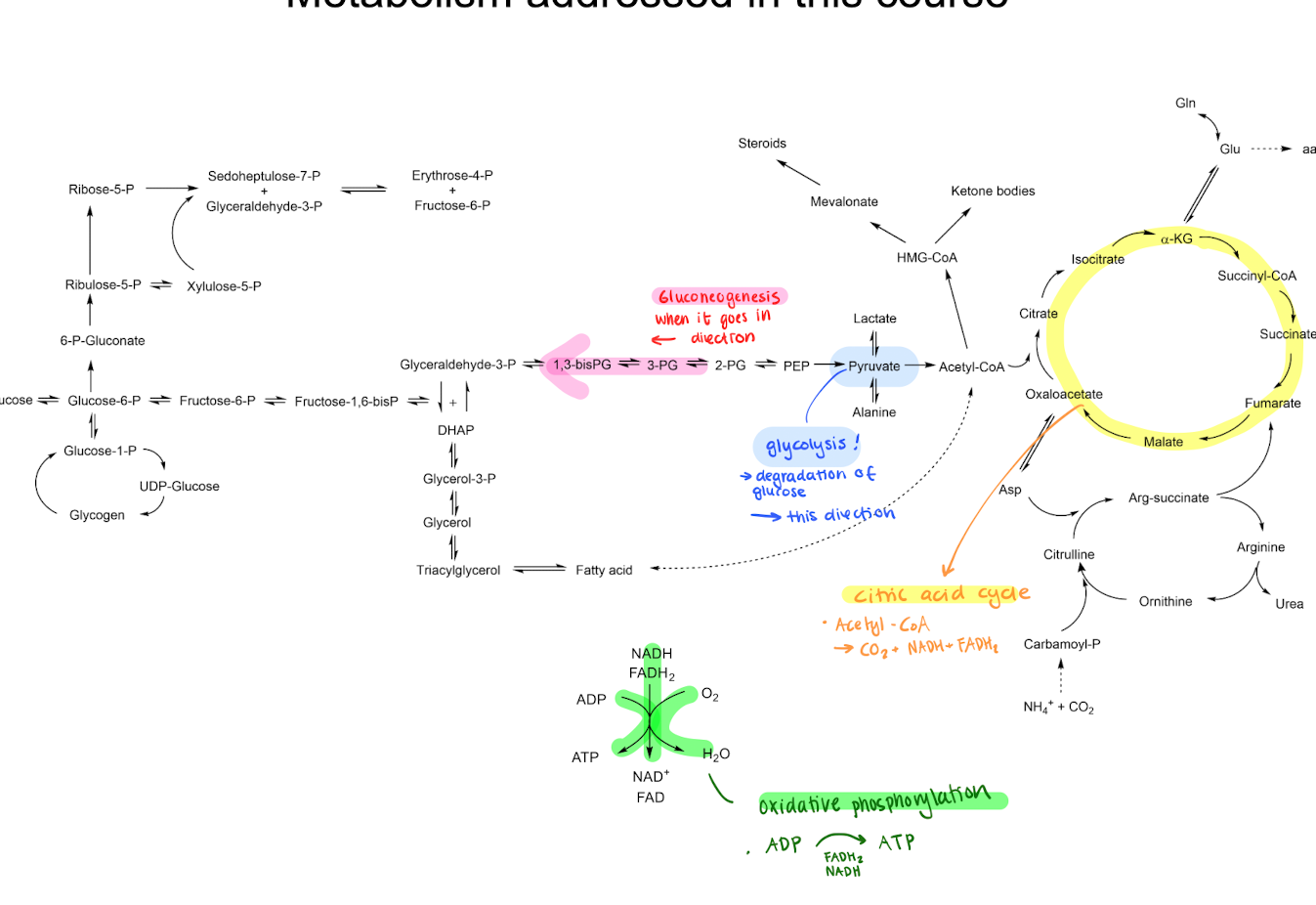
Glycolysis, basic structure, activation of glucose
Turning glucose (6C) into 2x Pyruvate (3C) through different reactions. This happens in the cytoplasm
aerobic : Crucial for pyruvate to go into citric acid cycle and oxidative phosphorylation inside of the mitocondia. (This generates more ATP)
anaerobic : Pyruvate turns into lactic acid.
1. Activate glucose by binding phosphate (costs 2 ATP), preventing glucose from leaving the cell.
2. Cleaving ATP to pyruvate
3. Recycle ATP + NADH to get 4 ATP
You should get 2 ATP out from these steps.

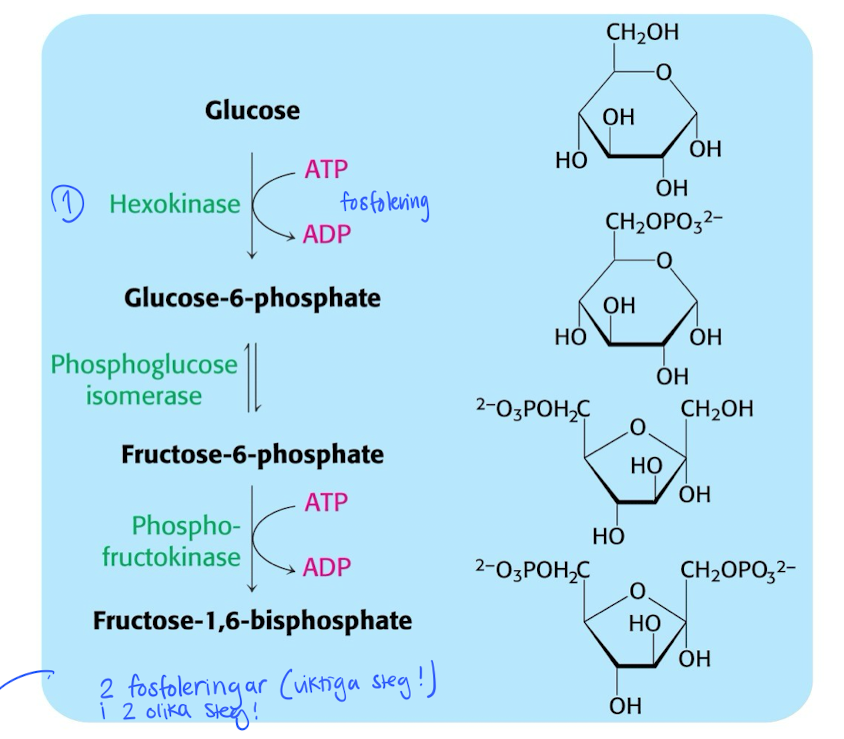
Glycolysis stage 1 : Glucose before cleavage
Activate glucose by 2 phosphorylation steps. Costs 2 ATP / glucose.
hexokinase gives negative charge to glucose → better kept inside the cell.
After that, the molecule (Fructose1,6-biphosphate) will be cleaved into G3P using aldolase.
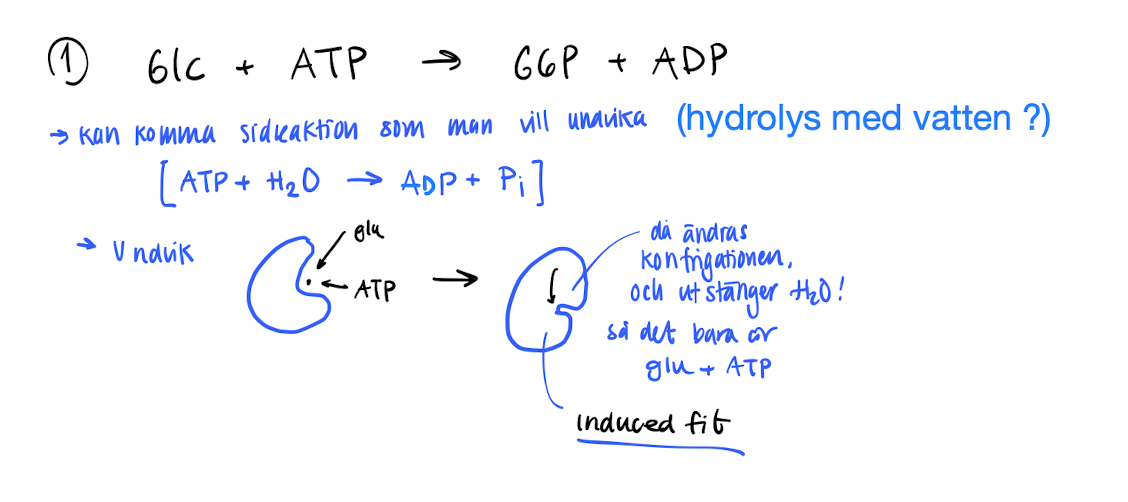
What can happen during the hexokinase phosphorylation?
Glc + ATP → G6P + ADP
A side reaction (hydrolysis) with water can occur, ATP + H2O → ADP + Pi
To combat this, hexokinase makes a induced fit when Glucose binds to the active site, changing the configuration and blocks H2O from entering.
Glycolysis stage 2: Cleavage of Fructose 1,6-biphsophate
Cleaved into 2 trioses using Aldolase → of those 2, only glyceraldehyde 3-phosphate (G3P) will continue to next step.
However, the other triose can convert ini G3P through triose phosphate isosmerase by forming an enediol intermediate.
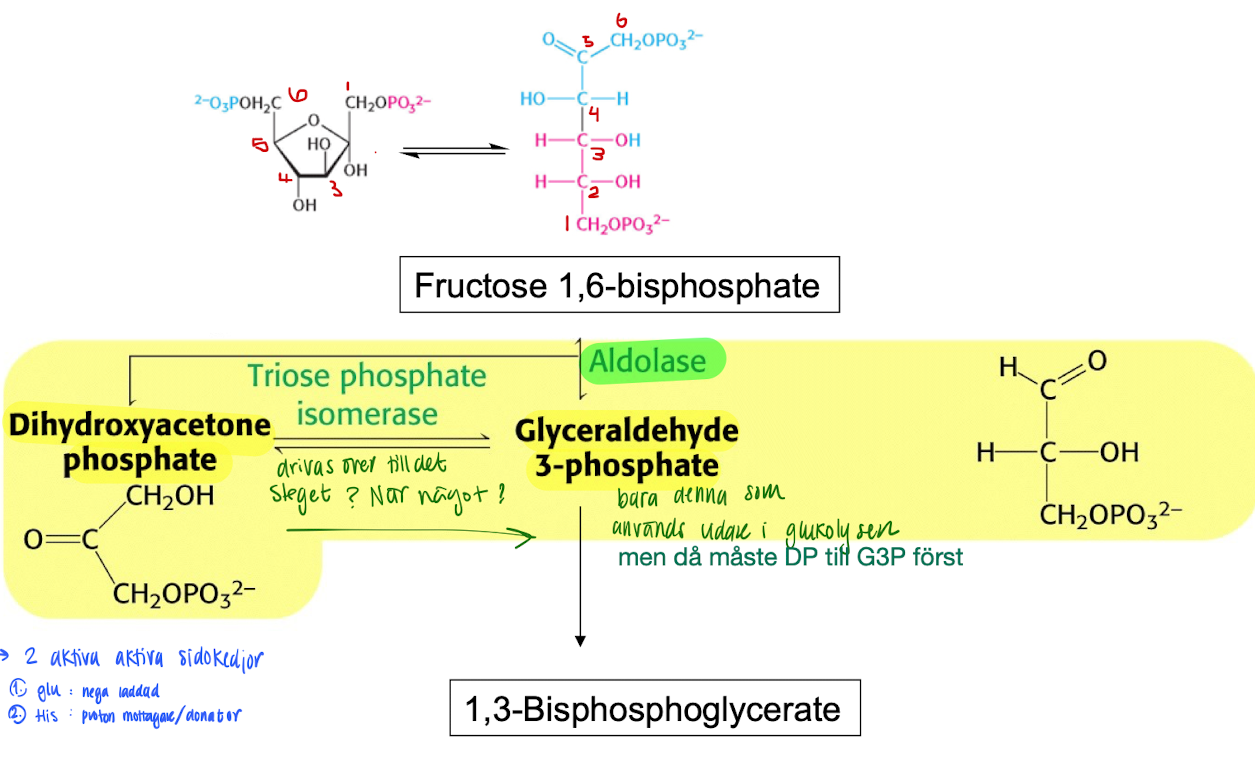
Glycolysis stage 2: oxidation of G3P
G3P is oxidized in steps to finally form pyruvate.
1 pyruvate → 2 ATP + 1 NADH
OBS! 1 glucose → 2 pyruvate, so the energy generated is doubled. So 1 glucose = 4 ATP.
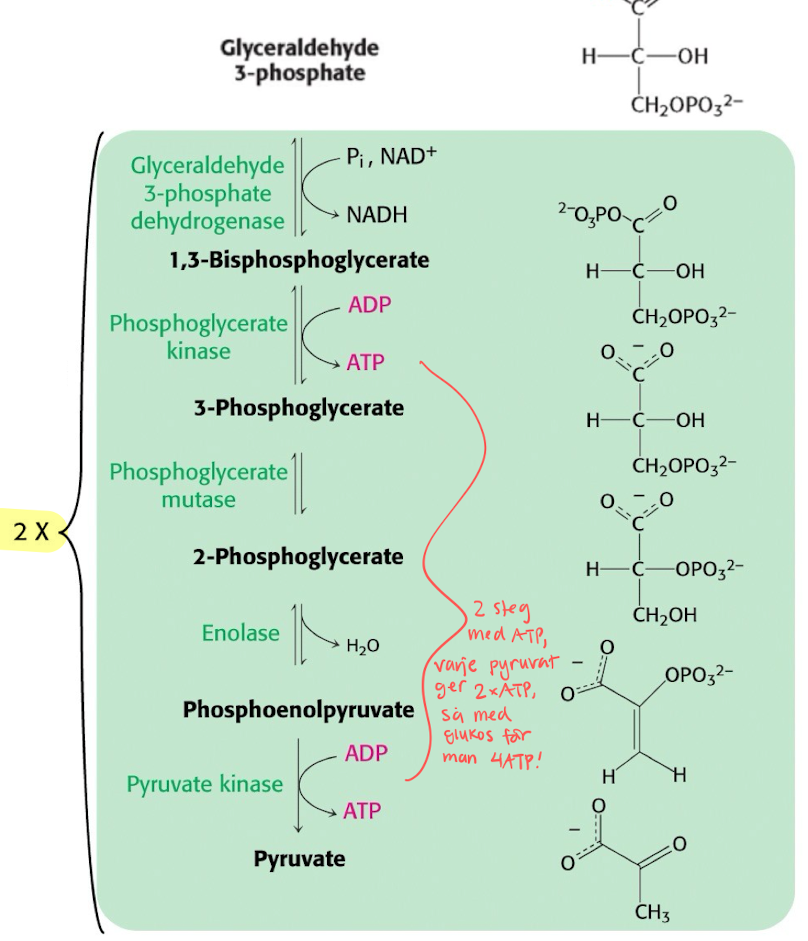
Amount of O2 will decide pyruvate’s reaction pathway
Gott om O2
den vanliga vägen till KREB, omvandlas till Acetyl-CoA och släpper en CO2.
O2 absent (Anoxia)
Turns into lactic acid? Using NADH as a electron donor to form NAD+.
O2 deficiency (Hypoxia) : only in yeast
Turns into Acetaldehyde (very reactive to other molecules, needs to get broken down) and later into Ethanol using NADH to form NAD+.
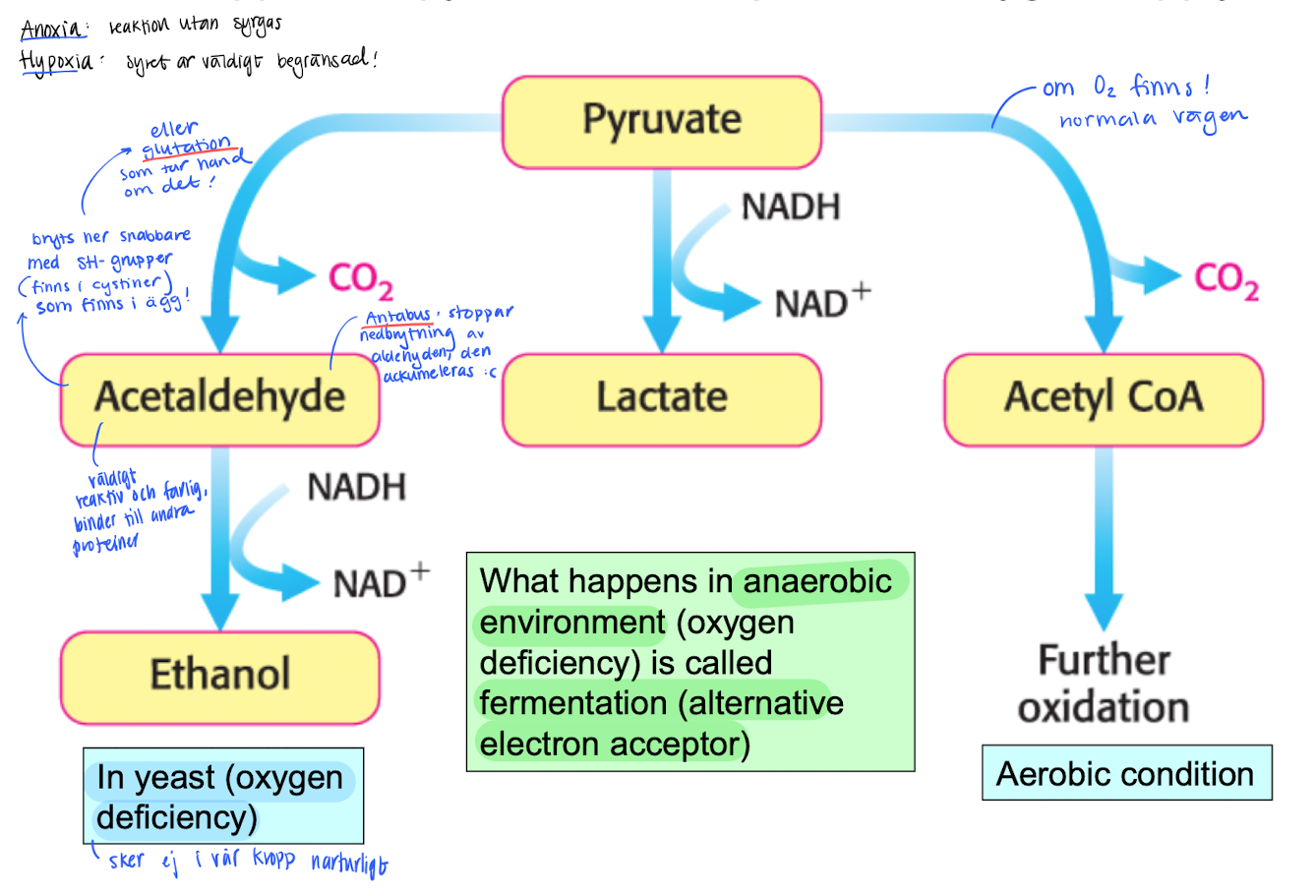
Why does Anoxia och Hypoxia reactions happen? (When O2 is lacking or completely absent)
For the glycolysis to continue, NAD+ needs to be quickly regenerated from NADH.
When O2 is present, it is used as a electron acceptor, when absent, the cells uses pyruvate instead (in muscles).
Pyruvate + NADH → Lactic acid + NAD+
Galactose (mjölksocker) and fructose in glycolysis
Galactose
Pathway is similar to glucose
Also gets activated by phosphorylation with ATP.
Fructose
Also gets activated by phosphorylation with ATP.
When glucose is rare, hexokinase can phosphorylate fructose to F6P directly (usually glucose does that).
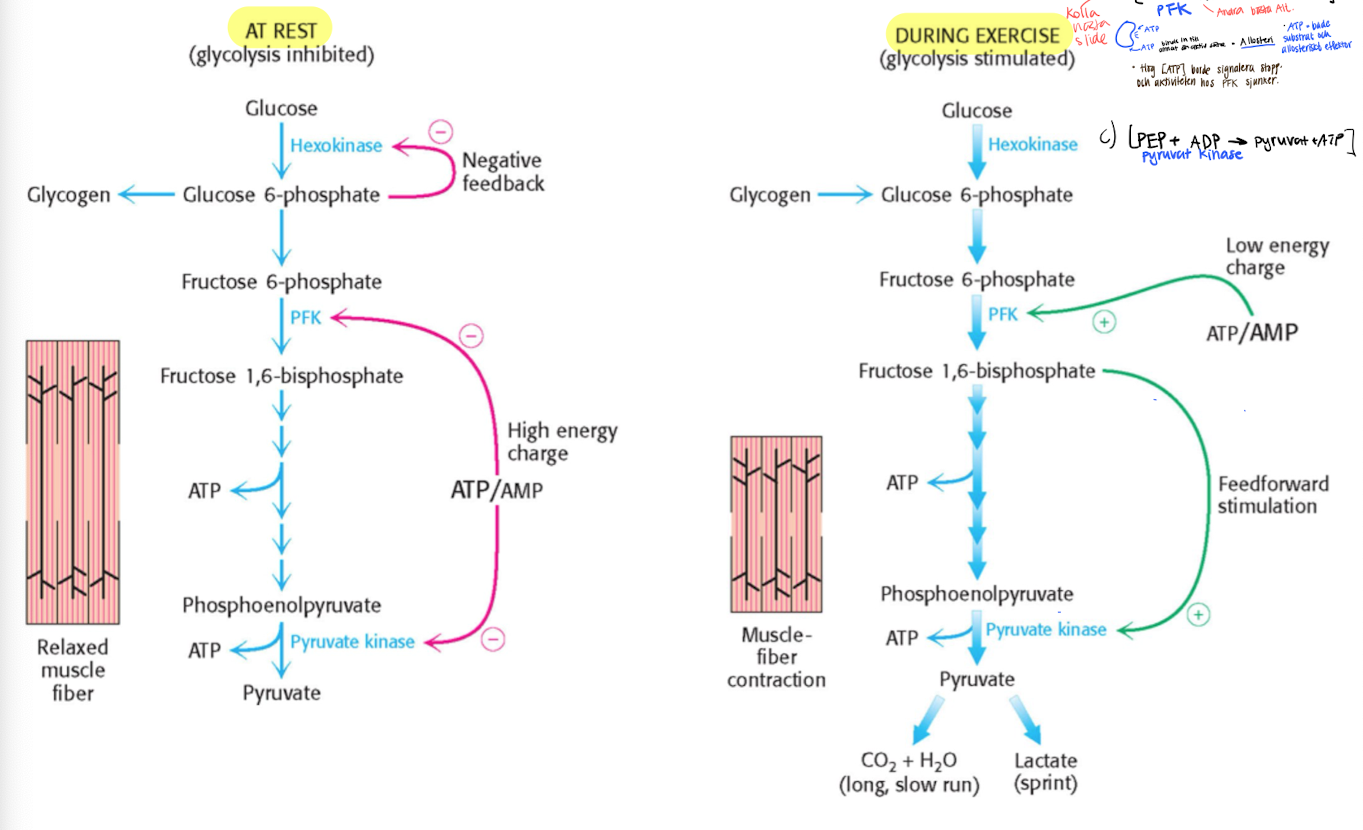
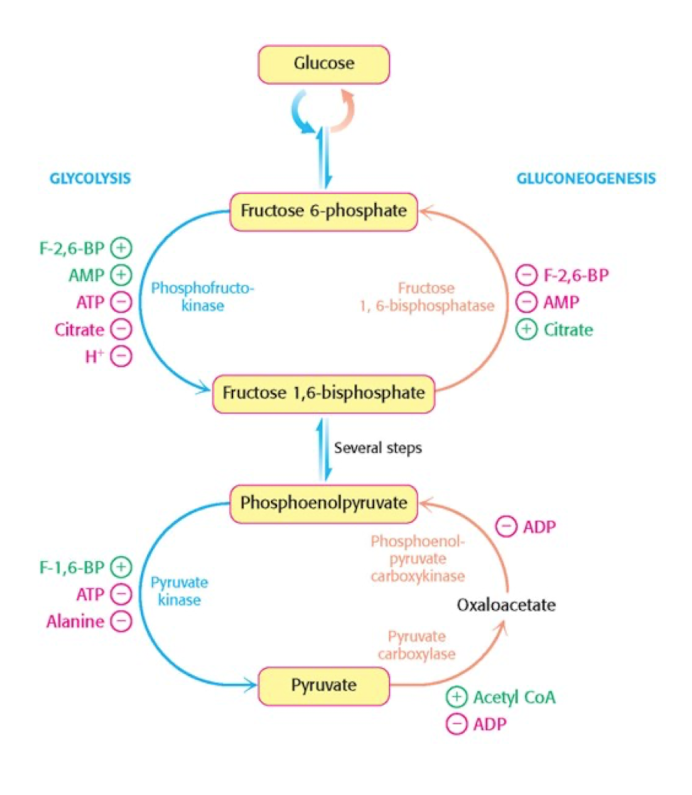
Control of glycolysis in muscles.
Vill kontrollera ireversibelt steg, vilket är steget som involverar PFK, vilket regurleras av mängden ATP genom allosteric regulation.
ATP funkar både som substrat och allosteriskt effektor.
High [ATP] → decreased PFK’s activity to bind F6P, low [ATP] during exercise will increase the activity.
F2,6BP also effective activator at low conc.
![<ul><li><p>Vill kontrollera<u> ireversibelt steg</u>, vilket är steget som involverar <span style="color: blue"><strong>PFK</strong></span>, vilket regurleras av mängden ATP genom allosteric regulation. </p></li><li><p>ATP funkar både som substrat och allosteriskt effektor. </p></li><li><p>High [ATP] → decreased PFK’s activity to bind F6P, low [ATP] during exercise will increase the activity. </p></li></ul><p></p><ul><li><p>F2,6BP also effective activator at low conc. </p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/ab0fdb6c-e3e3-4d2e-baa3-06e106bbcc5f.png)
what is GLUT ?
Different transport proteins to get glucose into the cell.
Different kinetic properties, adapted to functions in varying metabolic states.
GLUT receptors are on the surface of the cells.
Brukar märkas på tumörer eftersom de växer snabbt och tar näring genom glycogen, så tar emot jätte mycket glucose.