Final Exam - Microbiology
1/91
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
92 Terms
Each of the following organisms would be considered a microbe EXCEPT
Mushroom
Archaea differ from bacteria in that archaea
Have diverse cell wall compositions
Normal microbiota are typically found in and on all the following body locations EXCEPT the
Blood
Which of the following statements about the atom C is FALSE?
It has 12 neutrons in its nucleus
Antacids neutralize acid by the following reaction. Identify the salt in the following equation:
Mg(OH)2 + 2HCl → MgCl2 + H2O
MgCl2
Which of the following statements is false?
Water molecules are formed by hydrolysis
Which of the following is the type of bond holding K+ and I- ions in KI?
Ionic bond
Which molecule is composed of a chain of amino acids?
Protein
Which of the following pairs is mismatched?
safranin - acid dye
The purpose of a mordant in the Gram stain is to
Prevent the crystal violet from leaving the cells
Which microscope achieves the highest magnification and greatest resolution?
Electron microscope
Which of the following correctly traces the path of the light through the compound microscope?
Light source; condenser; specimen; objective lens; ocular lens
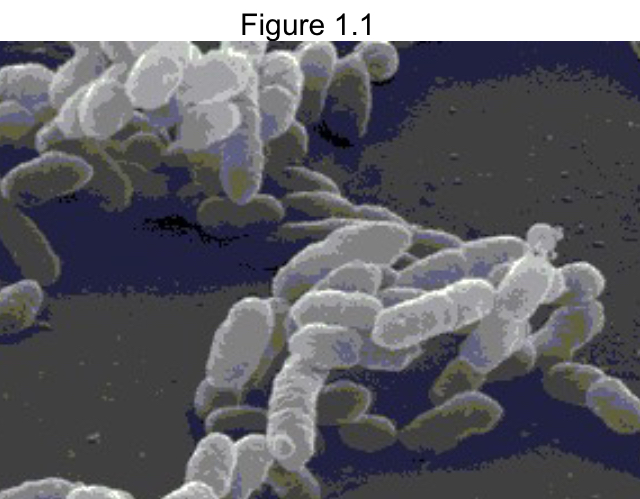
The bacterial shape of the cells in Figure 1.1 would best be described as
bacillus
Elements only achieve the full complement of electrons in outermost energy cells by donating or sharing electrons. T or F
False
Covalent bonds are always shared equally. T or F
False
A basic solution is expected to contain more hydrogen ions than hydroxyl ions.
False
In a completed Gram stain, gram-negative bacteria are colorless.
False
Both phase-contrast microscopy and differential interference contrast microscopy are used to view the internal structures of cells without staining.
True
The amateur scientist__________________________ made his own microscopes and first reported the existence of microbes.
Leeuwenhoek
A cell that contains a nucleus is called a(n) __________________________ cell.
eukaryotic
A scientist conducts experiments to test a(n)___________________.
hypothesis
A __________________ is a community of microbes growing on surfaces.
biofilm
An atom or molecule becomes a(n) _____________ when it loses an electron to a more electronegative molecule.
Cation
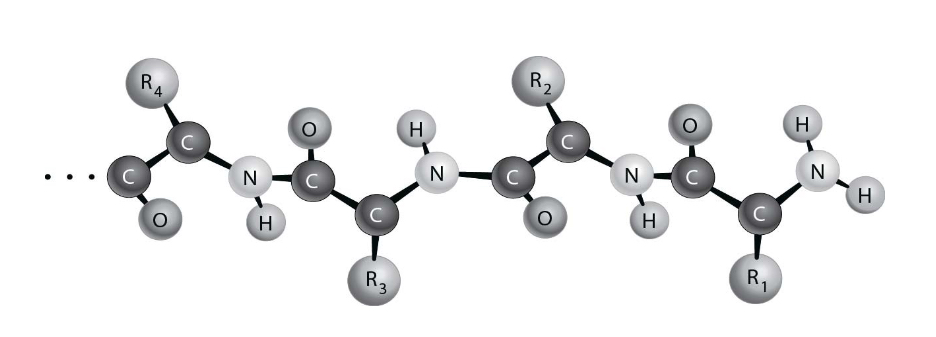
Figure 2.2 depicts the_________________structure of a protein.
Primary
A chemical reaction that traps energy within newly formed chemical bonds is an ________________ reaction.
Endothermic
Which of the following statements about bright field microscopy are true?
Bright field microscopy is the simplest and most common form of my microscopy.
Knowing just the atomic mass of an element allows inferences about which of the following?
The number of protons plus neutrons in the element
When do opportunistic pathogens tend to cause disease?
When the host is weakened
Which of the biomolecules is incorrectly matched with its building block?
Carbohydrate: Polysaccharide
What is the order of the taxonomic hierarchy from least specific to most specific?
domain, kingdom, phylum, class, order, family, genus, species
Van der Waals interactions result when
electrons are not symmetrically distributed in a molecule.
Electrolytes
include acids, bases, and salts, are ionic compounds dissolved in solution and are involved in regulating the nervous system, heartbeat, blood volume and water balance in the body.
A given solution contains 0.00001 (10-4) moles of hydrogen ions (H+) per liter. Which of the following best describes this solution?
It has a pH of 4
dentify the following reaction: HCl + NaHCO3 → NaCl + H2CO3
Exchange reaction
The slight negative charge at one end of one water molecule is attracted to the slight positive charge of another water molecule. What is this attraction called?
A hydrogen bond
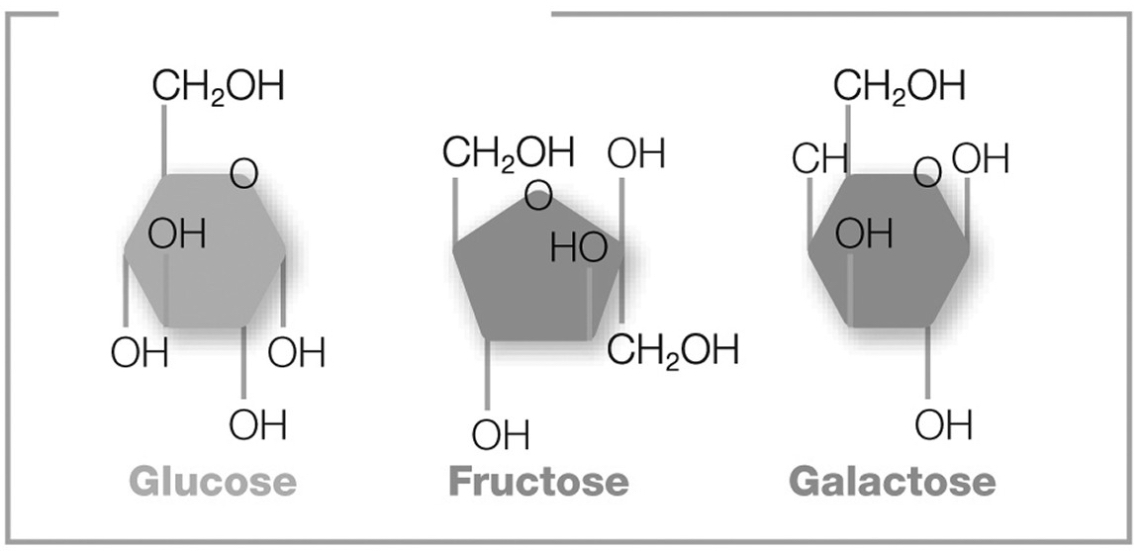
The pictured molecules both contain six carbon atoms, twelve hydrogen atoms, and six oxygen atoms (C6H12O6). However, these atoms are arranged differently in each molecule. What are these molecules called?
Isomers
Microbes and humans have evolved a variety of ________ relationships, including ________ where microbes help the host.
symbiotic; mutualism
________ predict what happens, while ________ explain how and why something occurs.
Laws; theories
Why can't prokaryotic species be defined as a group of similar organisms that could sexually reproduce together?
Bacteria reproduce asexually.
Which of the following is true about bioremediation?
Nitrogen, sulfur, phosphate, and sometimes iron supplements are added to the spill zone to encourage microbial growth.
What is the difference between covalent bonds and ionic bonds?
Covalent bonds involve the sharing of electrons between atoms; ionic bonds involve the electrical attraction between atoms.
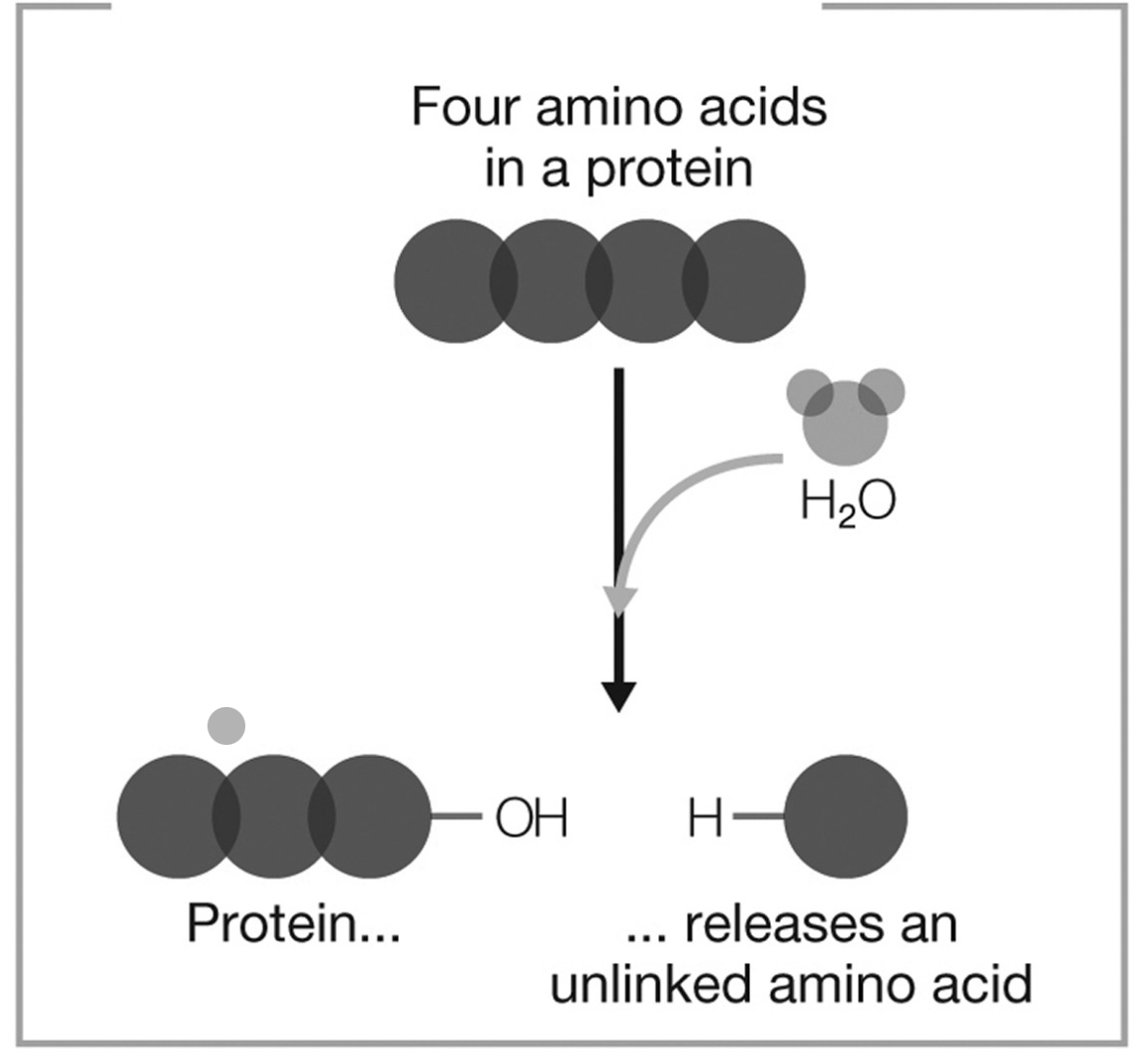
What type of reaction does the figure show?
decomposition and hydrolysis
________ showed that biogenesis is responsible for the propagation of life.
Louis Pasteur
Why is each element unique and different from other elements with respect to its chemical properties?
Each element has a unique number of protons.
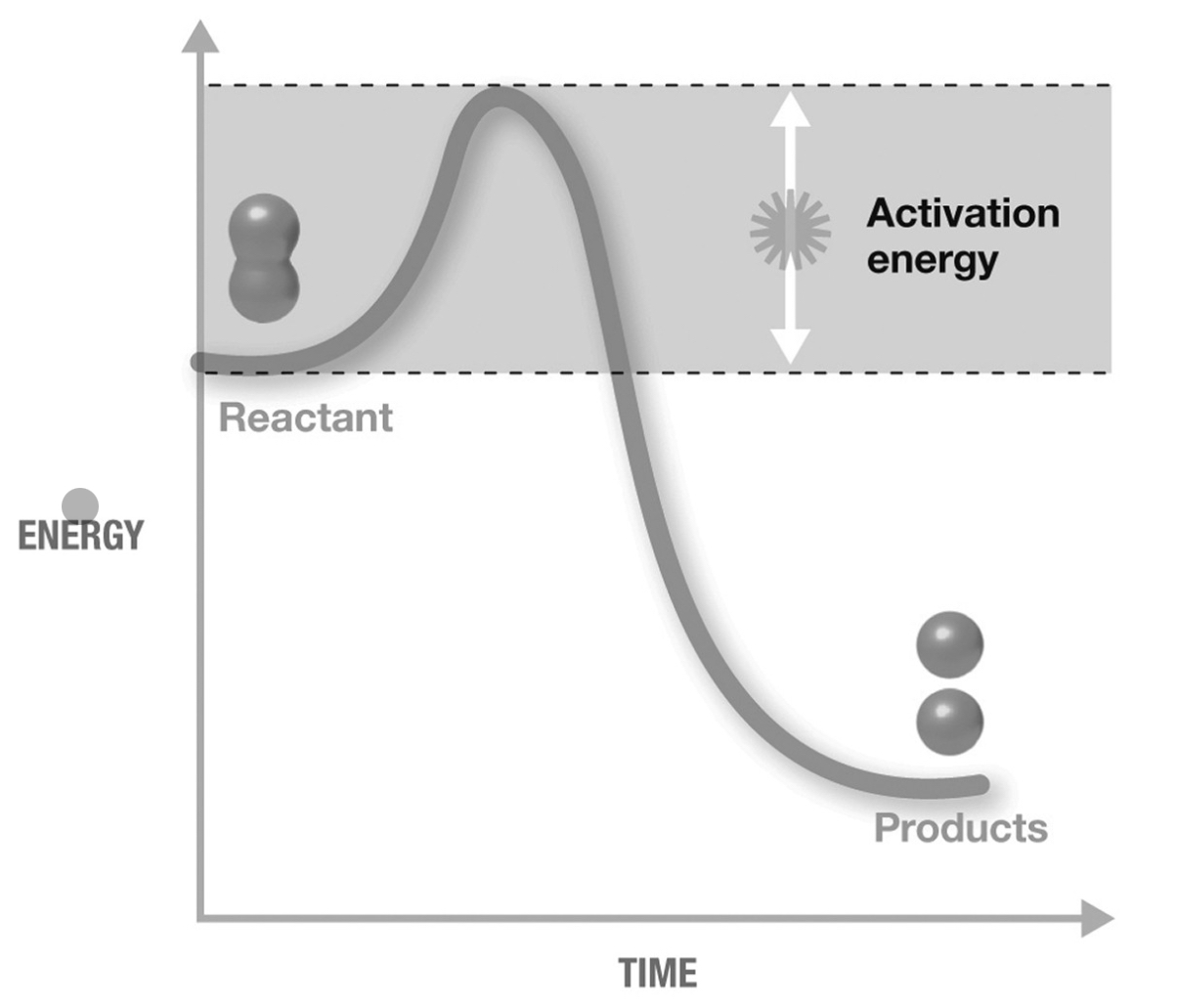
The figure shown is an exergonic reaction because
the products have a lower final energy than the reactants.
Which of the following statements is true about buffer solutions?
They maintain a relatively constant pH when either acids or bases are added to them.
The partial negative charge in a molecule of water occurs because
The electrons shared between the oxygen and hydrogen atoms spend more time around the oxygen atom’s nucleus than around the hydrogen atom’s nucleus.
Robert Koch helped establish the germ theory of disease by discovering that anthrax was caused by a disease. After he isolated and purified the same bacteria from several diseased animals, what would be the next step in order to show that this bacteria caused anthrax?
introduce the bacteria into a new mouse to see if it established the same infection
Which of the following can be determined using simple stains?
size, shape and cellular arrangement
Which particle is described incorrectly?
Proton: Found in shells orbiting the nucleus
Which of the following is the type of bond holding K+ and I- ions in KI?
ionic bond
A covalent bond is likely to be polar when
one of the atoms sharing electrons is much more electronegative than the other atom.
Hydrophobic substances such as vegetable oil are
nonpolar substances that repel water molecules.
The goal of the streak plate technique is to
isolate a pure culture for study from a single colony.
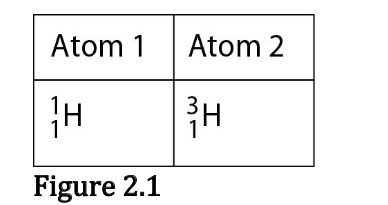
Which of the following best describes the relationship between the atoms described in Figure 2.1?
They are isotopes.
Biofilms allow microbes to coordinate responses within an environment, making the community much more durable than single free-floating bacteria. T or F
True
Florence Nightingale investigated processes for aseptic surgery and her work in the 1860s proved that sterilizing instruments, and sanitizing wounds with carbolic acid encouraged healing and prevented pus formation. T or F
False
A cation forms when an atom loses one or more negatively-charged electrons. T or F
True
Knowing the Gram property of a specimen has important clinical implications, including potential pathogenic features of the organism, and what antibiotics might be most effective in combating it.
True
All chemical reactions are, in theory, reversible. T or F
True
Simple staining techniques use one dye. T or F
True
Endergonic reactions make products with a lower final energy than the reactants and use more energy than is released. T or F
False
There are some forms of life on Earth that can survive without water. T or F
False
A chemical reaction in which a water molecule is a reactant is known as a(n) (dehydration/hydrolysis) reaction.
Hydrolysis
The folding of a polypeptide into a three-dimensional shape is its (secondary/tertiary/quaternary) structure.
Tertiary
Each of the following statements concerning the gram-positive cell wall is true EXCEPT
it protects the cell in a hypertonic environment.
Which of the following have a cell wall?
fungi
By which of the following mechanisms can a cell transport a substance from a lower to a higher concentration?
active transport
A gram-positive bacteria suddenly acquires resistance to the antibiotic methicillin. This trait most likely occurred due to
conjugation.
Which of the following statements is INCORRECT regarding prokaryotic cells?
They lack a plasma membrane.
Functions of the glycocalyx include all of the following EXCEPT
binary fission.
Which structure acts like an “invisibility cloak” and protects bacteria from being phagocytized?
capsule
The difference between simple diffusion and facilitated diffusion is that facilitated diffusion
requires transporter proteins.
The terms “run” and “tumble” are generally associated with
taxic movements of the cell.
Which one of the following pairs is mismatched?
ribosomes - protein storage
Examination of a cell by transmission electron microscopy reveals a high density of ribosomes in the cytoplasm. This observation suggests that this cell is actively producing large amounts of which of the following molecules?
proteins
A cell with a predominance of smooth endoplasmic reticulum is likely specialized to ________.
synthesize large quantities of lipids
Which of the following is the most common pathway taken by a newly synthesized protein that will be secreted by a cell?
Rough ER → Golgi → transport vesicle → plasma membrane
Motor proteins provide for molecular motion in cells by interacting with what types of cellular structures?
components of the cytoskeleton
A characteristic 9 + 2 arrangement of microtubules, consisting of nine doublets of microtubules surrounding a pair of single microtubules is associated with ________.
eukaryotic flagella and motile cilia
Compared to eukaryotes, prokaryotes are ________.
simpler morphologically, but not more evolutionarily primitive
While examining a rock surface, you have discovered an interesting new organism. Which of the following criteria will allow you to classify the organism as belonging to Bacteria but not Archaea or Eukarya?
Cell walls are made primarily of peptidoglycan.
Which of the following statements about the cytoskeleton is true?
Movement of cilia and flagella is the result of motor proteins causing microtubules to move relative to each other.
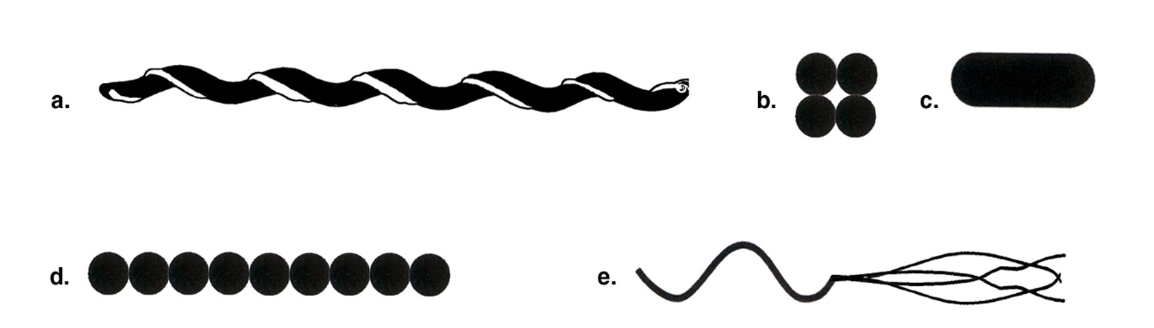
Which drawing in Figure 4.1 is a tetrad?
B
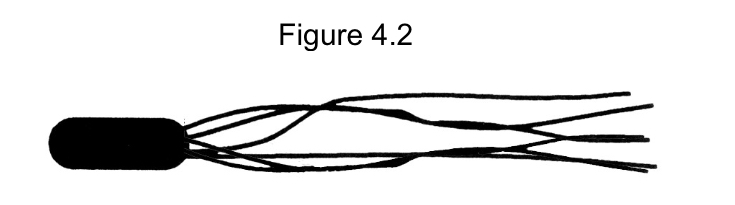
Which of the following terms best describes the cell in Figure 4.2?
lophotrichous flagella
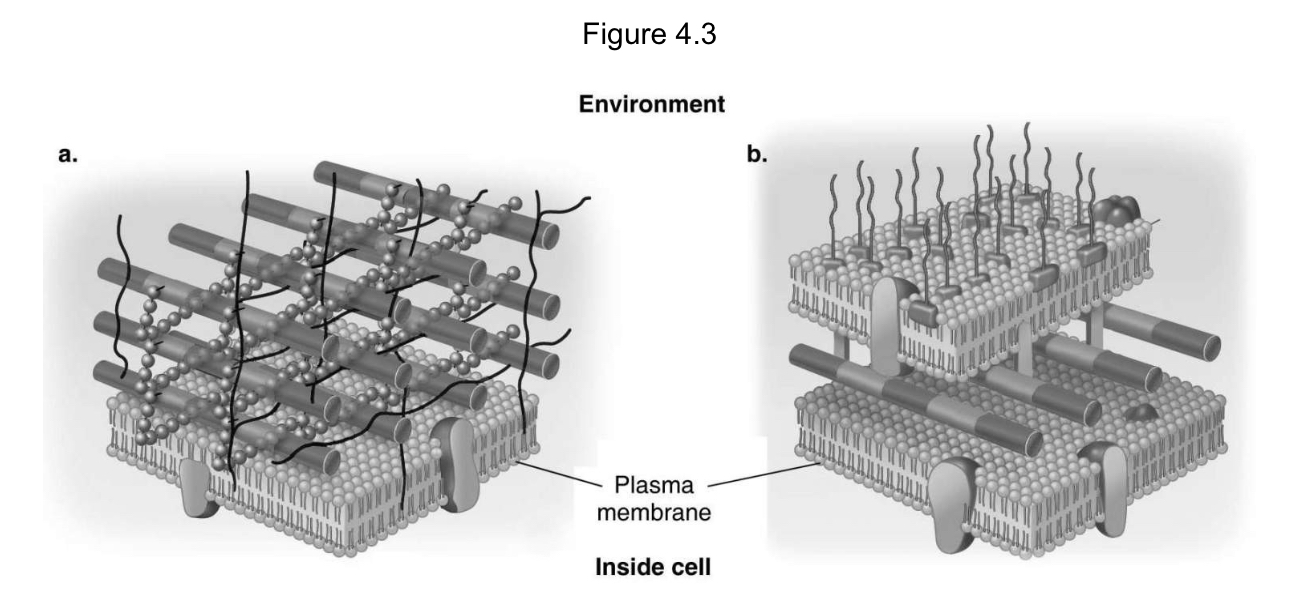
In Figure 4.3, which diagram of a cell wall is a gram-negative cell wall?
B
The cell walls of bacteria are responsible for the shape of the bacteria and the difference in the Gram stain reaction. T or F
True
Cells placed in a hypotonic solution tend to lose water due to osmotic pressure. T or F
False
Small, hydrophobic molecules pass through a cell membrane most easily by diffusion. T or F
True
Endospores are a reproductive structure. T or F
False
Many enzymes in both prokaryotic and eukaryotic cells are compartmentalized within organelles.
False
Which of the following have a cell wall?
fungi