Pulmonary Function Testing and Disorders Overview
1/190
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
191 Terms
What does Pulmonary Function Testing (PFT) / Spirometry measure?
Airflow in the lung.
What is Forced Vital Capacity (FVC)?
The total volume of air exhaled during a forced breath.
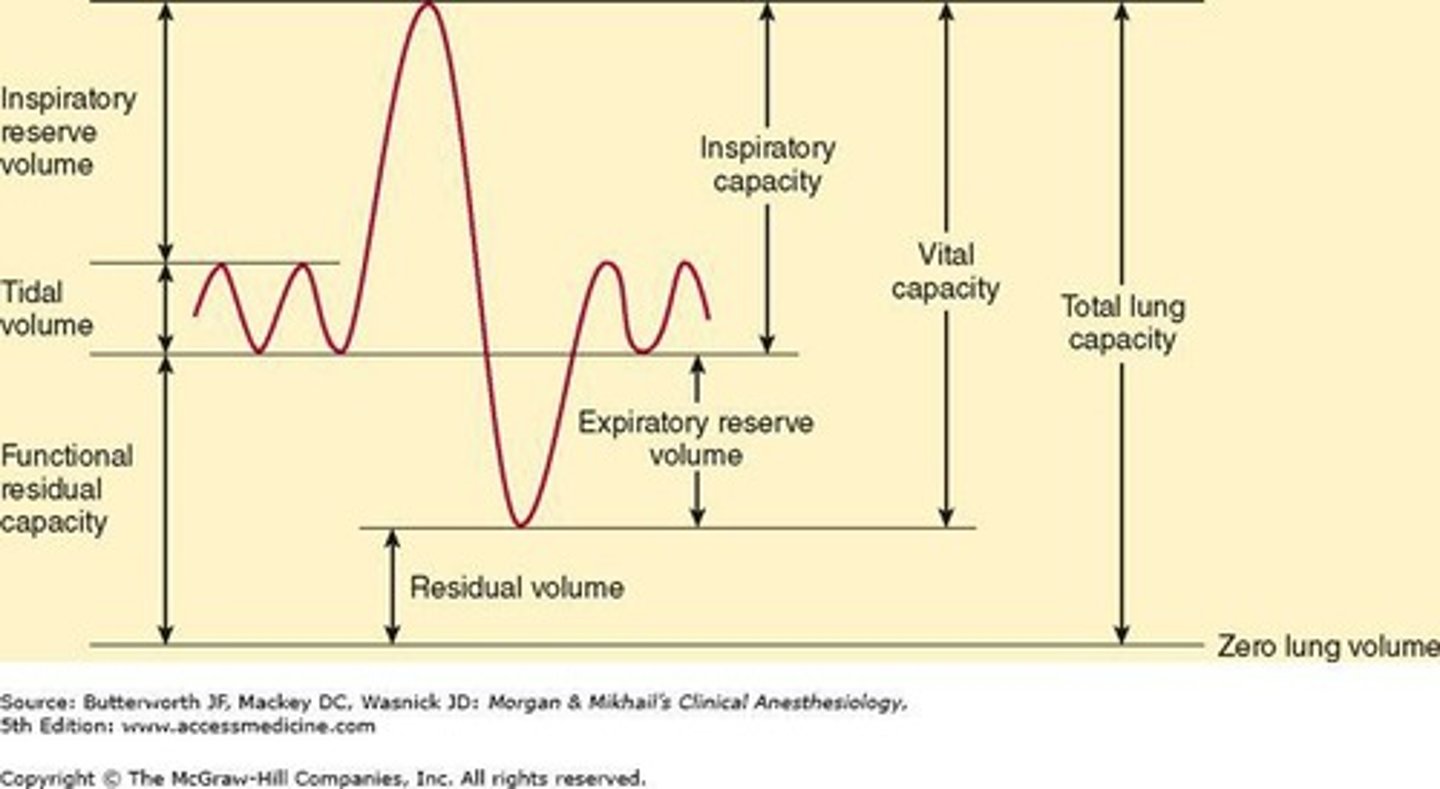
What does Forced Expiratory Volume in 1 second (FEV1) indicate?
The volume of air exhaled in the first second, serving as a reliable index of obstructive airway disease.
What is the normal range for the FEV1/FVC ratio?
Greater than 75%.
What does diffusion capacity measure in pulmonary function tests?
The ability of alveolar gases to diffuse into the capillary.
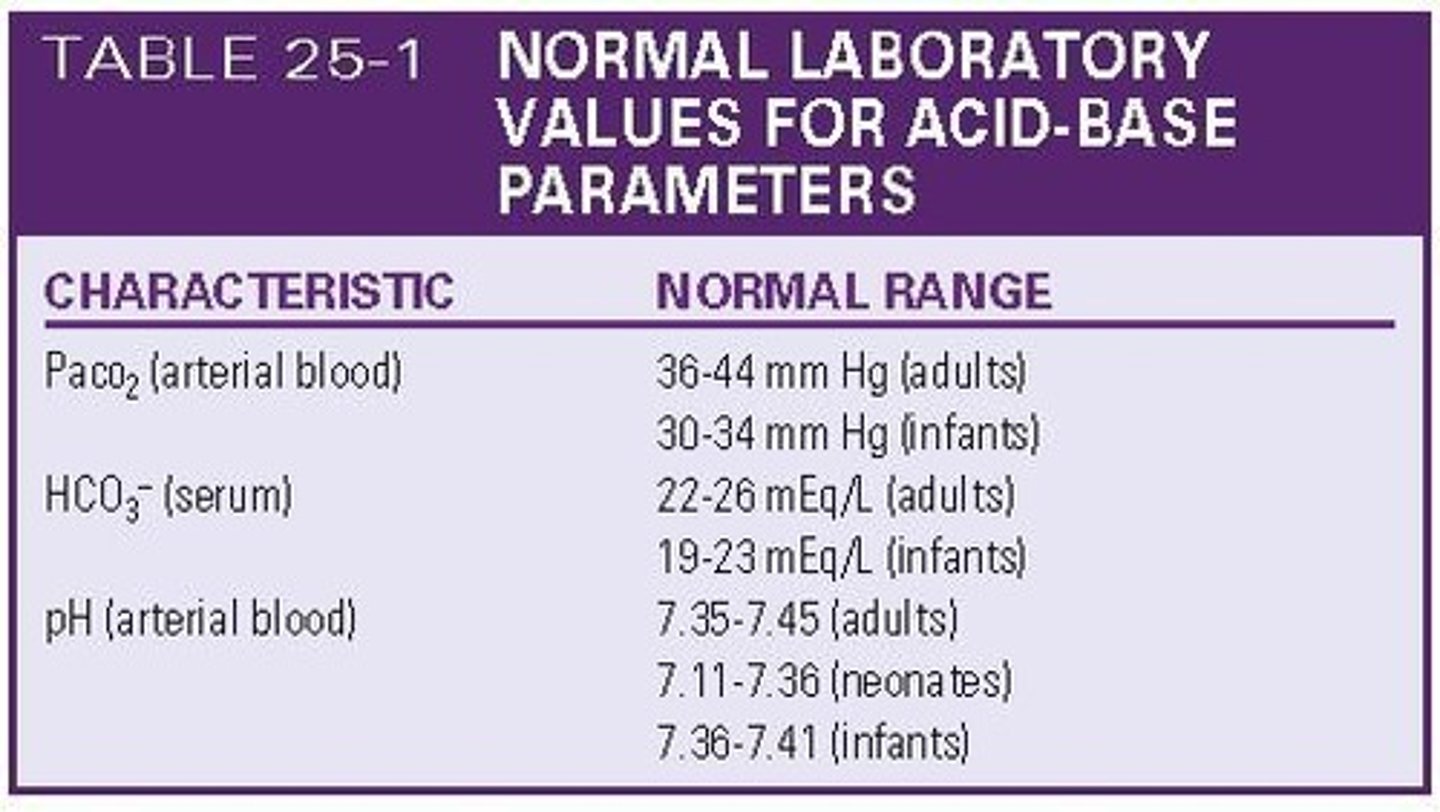
What are the normal values for arterial blood gas (ABG) measurements?
PaO2: 80-100 mmHg, PaCO2: 34-45 mmHg, PaHCO3-: 22-26 mEq/L, O2 Saturation: 96-100%.
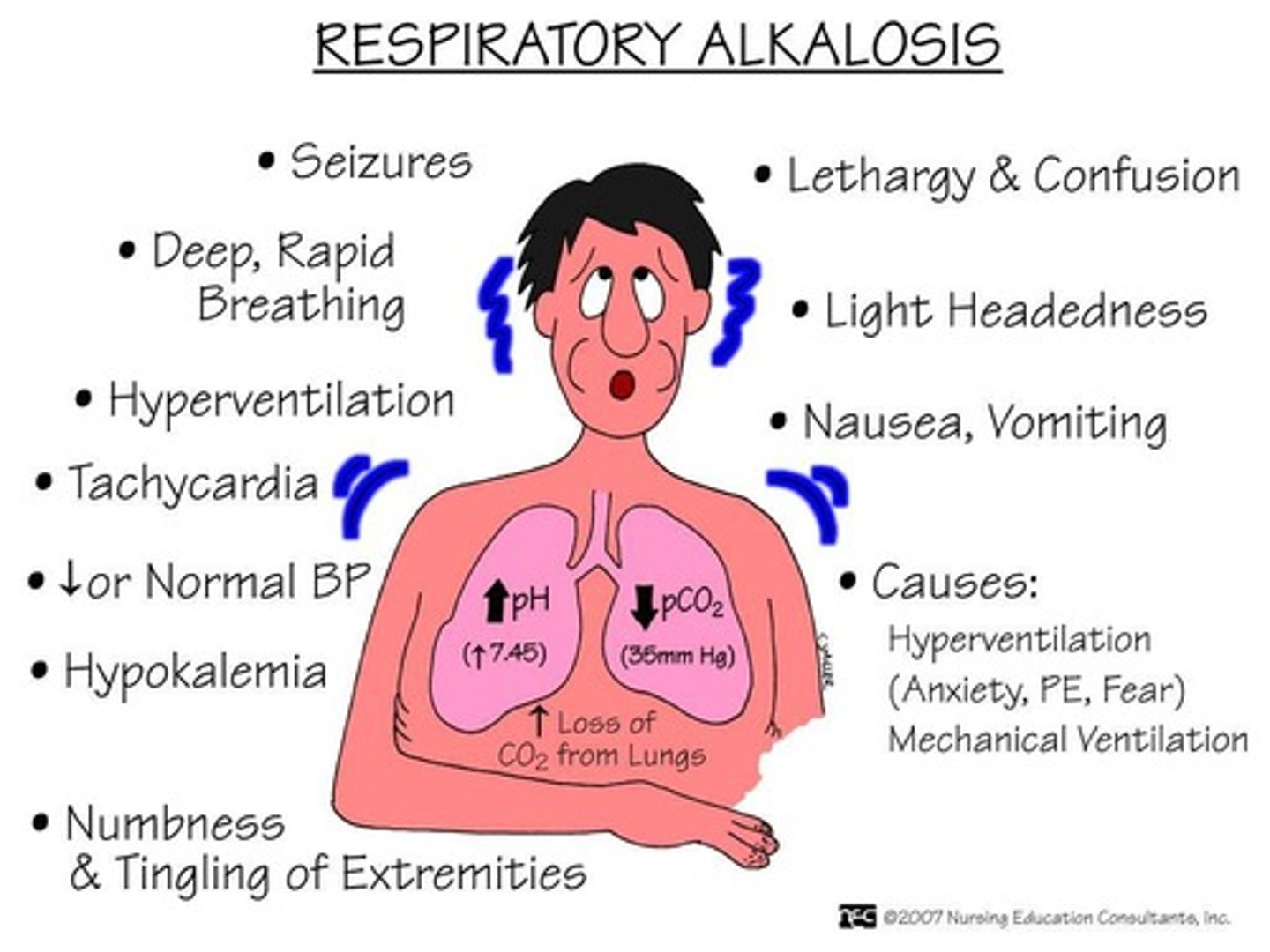
What is the normal pH range for arterial blood gas?
7.35-7.45.
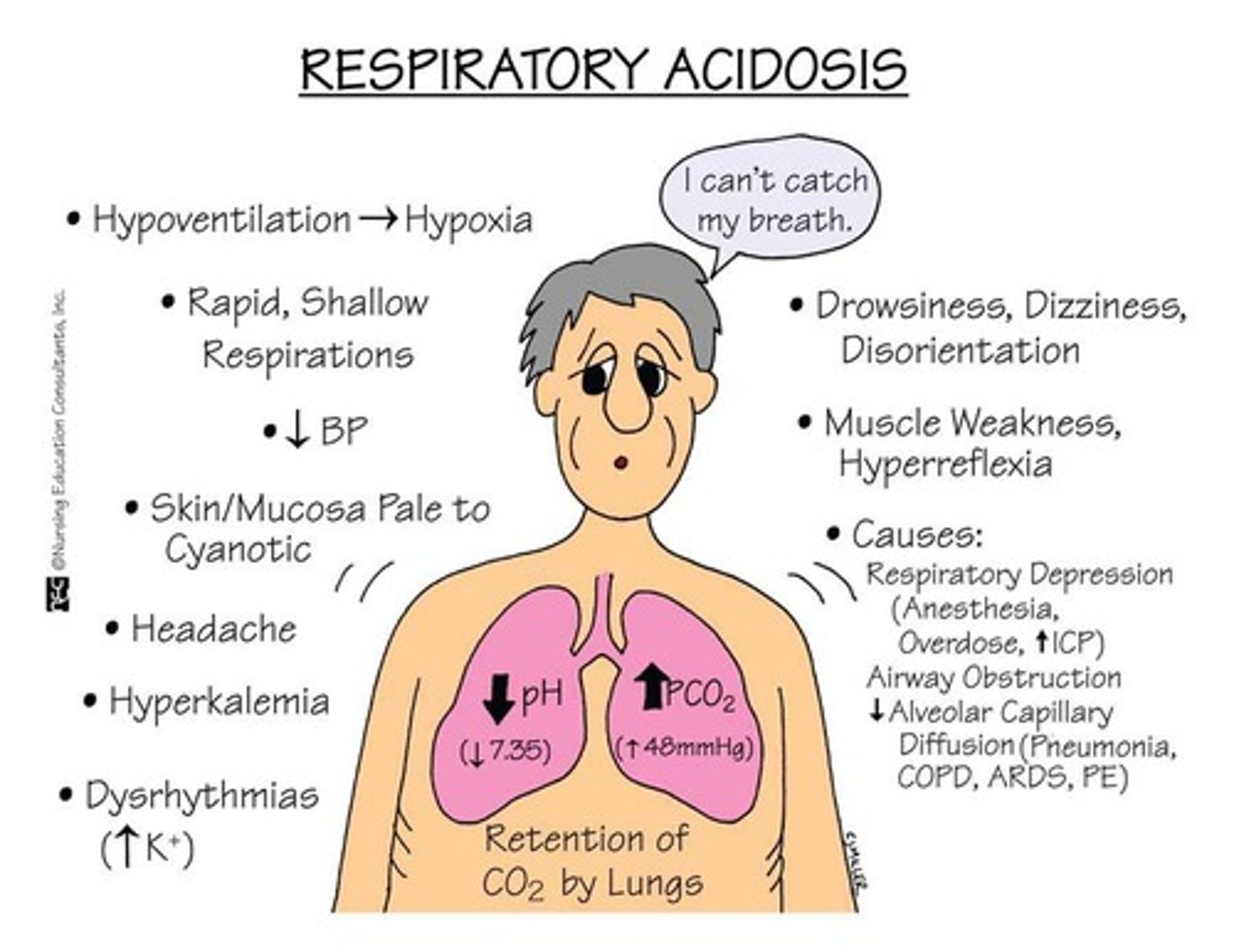
What indicates acidosis in arterial blood gas measurements?
A pH less than 7.35.
What indicates alkalosis in arterial blood gas measurements?
A pH greater than 7.45.
What is a characteristic finding in obstructive pulmonary disorders?
Increased resistance to airflow.
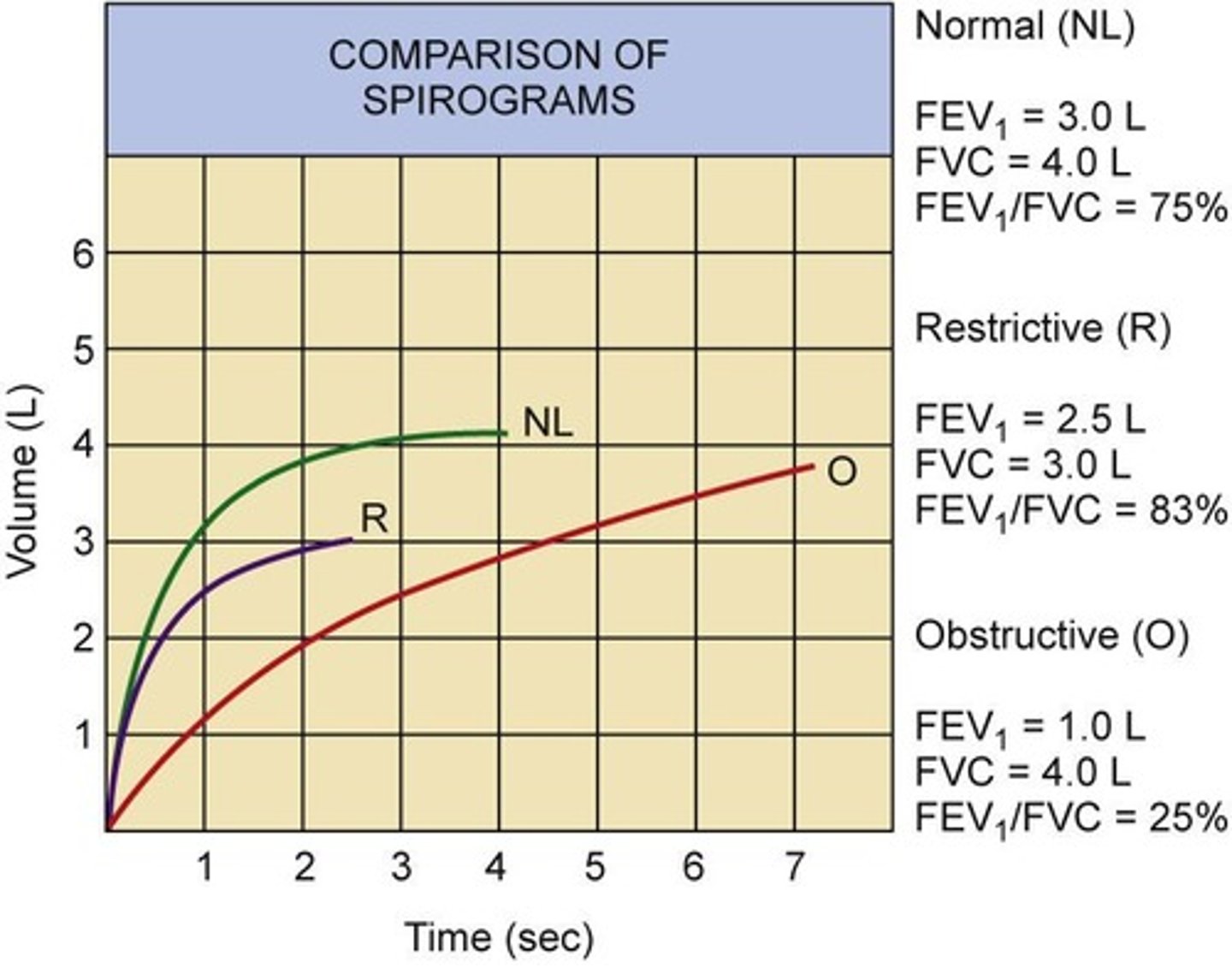
What FEV1/FVC ratio indicates an obstructive disorder?
Less than 70%.
What indicates a positive bronchodilator response in spirometry?
An improvement in FEV1 of more than 15% after bronchodilator use.
What are the characteristics of restrictive pulmonary disorders?
Decreased lung expansion, with normal FEV1/FVC ratio since both are reduced.
What happens to vital capacity (VC) in restrictive pulmonary disorders?
There is a decrease in vital capacity.
What is the effect of restrictive disorders on arterial blood gas (ABG) values?
Decreased PaO2, normal or decreased PaCO2, and increased pH (alkalosis).
What are the two classifications of obstructive pulmonary disorders?
Obstruction from conditions in the wall of the lumen and obstruction resulting from increasing pressure around the outside of the airway lumen.
What is asthma characterized by?
Reversible airway obstruction, airway inflammation, and increased airway responsiveness.
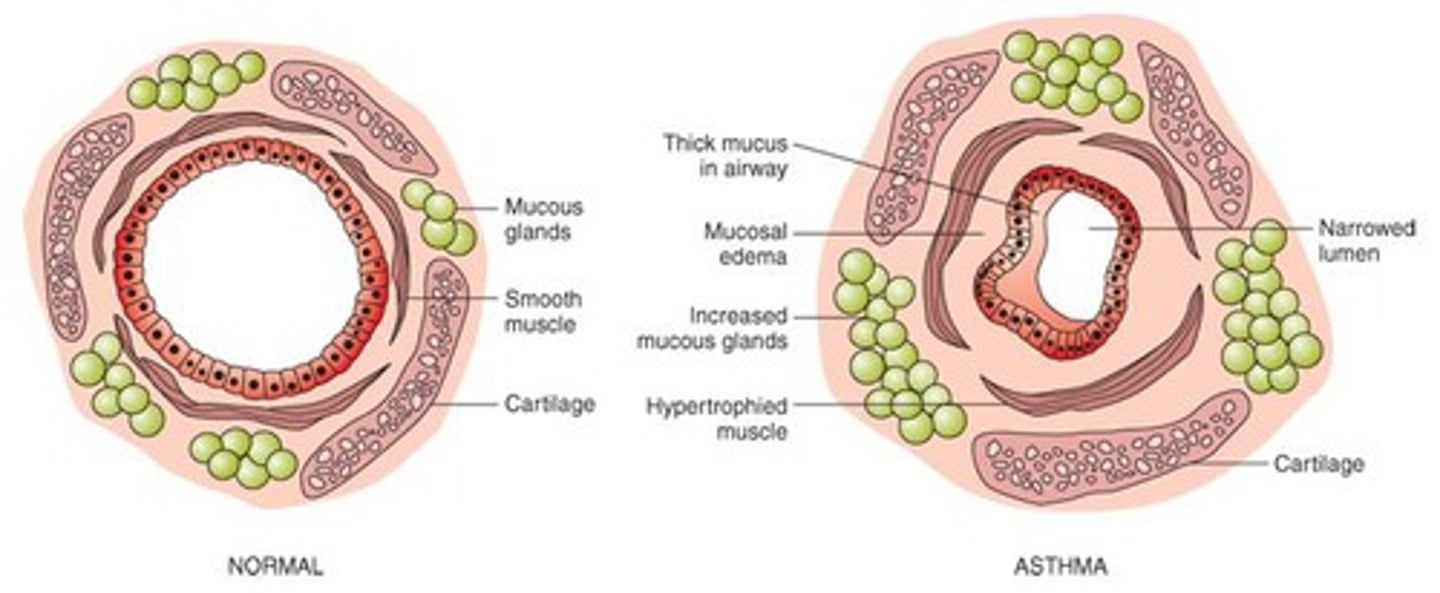
What are the two types of asthma?
Intrinsic (non-allergic, adult onset) and extrinsic (allergic, pediatric onset).
What is the etiology of intrinsic asthma?
Develops in middle age with no history of allergies, often triggered by respiratory infections or psychological factors.
What is the etiology of extrinsic asthma?
An IgE-mediated response leading to mast cell activation and inflammatory cell infiltration.
What are common clinical manifestations of asthma?
Wheezing, chest tightness, dyspnea, cough, and increased sputum production.
What is a common radiological finding in asthma patients?
Hyperinflated chest on X-ray.
What physical exam finding is often noted in asthma patients?
Decreased breath sounds.
What are the clinical manifestations of a severe asthma attack?
Use of accessory muscles of respiration, intercostal retractions, distant breath sounds with inspiratory wheezing, orthopnea, agitation, tachypnea (>30 breaths/min), tachycardia (>120 beats/min), pulsus paradoxus, and PEFR <80 L/min.
Why is wheezing not a good indicator of airflow in asthma?
Wheezing may not accurately reflect the severity of airflow obstruction.
What radiographic finding is associated with asthma?
Hyperinflation with flattening of the diaphragm.
What are the key findings in a sputum examination for asthma?
Charcot-Leyden crystals, eosinophils, and Curschmann spirals.
What pulmonary function test results indicate asthma?
Decreased forced expiratory volumes and a significant change in the FEV1/FVC ratio after bronchodilator administration.
What does ABG indicate during a mild asthma attack?
ABG is typically normal during mild attacks.
What ABG changes occur as bronchospasm increases in severity?
Respiratory alkalosis and hypoxemia, with potential PaCO2 elevation indicating worsening condition.
What laboratory findings are expected in a CBC for asthma patients?
Elevated WBCs and eosinophils.
What are the primary treatment strategies for asthma?
Avoid triggers, environmental control, preventive therapy, and desensitization.
What medications are commonly used to treat asthma?
O2 therapy, small-volume nebulizers, B2 agonists, corticosteroids, leukotriene modifiers, and mast cell inhibitors.
What defines chronic bronchitis?
A chronic or recurrent productive cough lasting more than 3 months for 2 or more successive years.
What is the male to female ratio for chronic bronchitis?
1:2 male to female ratio.
What age group is most commonly affected by chronic bronchitis?
Individuals over 30 to 40 years old.
What is the pathogenesis of chronic bronchitis?
Chronic inflammation and swelling of the bronchial mucosa, elevated IL8 levels, increased bronchial wall thickness, and hyperplasia of bronchial mucous glands.
What complications arise from chronic bronchitis?
Ventilation-perfusion mismatch leading to hypoxemia, hypercarbia, pulmonary hypertension, and potential right-sided heart failure.
What is the role of IL8 in chronic bronchitis?
Elevated IL8 levels recruit neutrophils, contributing to inflammation and airway obstruction.
How does chronic bronchitis affect oxygenation?
Inflammation and mucus production prevent proper oxygenation and increase airway obstruction.
What is the significance of mucus plugs in chronic bronchitis?
Mucus plugs obstruct airflow and contribute to respiratory distress.
What is cor pulmonale in the context of chronic bronchitis?
Right-sided heart failure resulting from pulmonary hypertension due to chronic bronchitis.
What lifestyle modifications are recommended for chronic bronchitis patients?
Avoiding smoking, second-hand smoke, and other respiratory irritants.
What is the relationship between chronic bronchitis and emphysema?
Chronic bronchitis is often paired with emphysema, leading to persistent and irreversible lung damage.
What is the last stage of chronic bronchitis characterized by?
Destruction of bronchial walls leading to dead space/emphysema.
What results from the destruction of bronchial walls in chronic bronchitis?
Dilation of airway sacs, known as bronchiectasis.
What complications can arise from dilated sacs in chronic bronchitis?
Pools of infected secretion that can cause further infection spreading to adjacent lung fields.
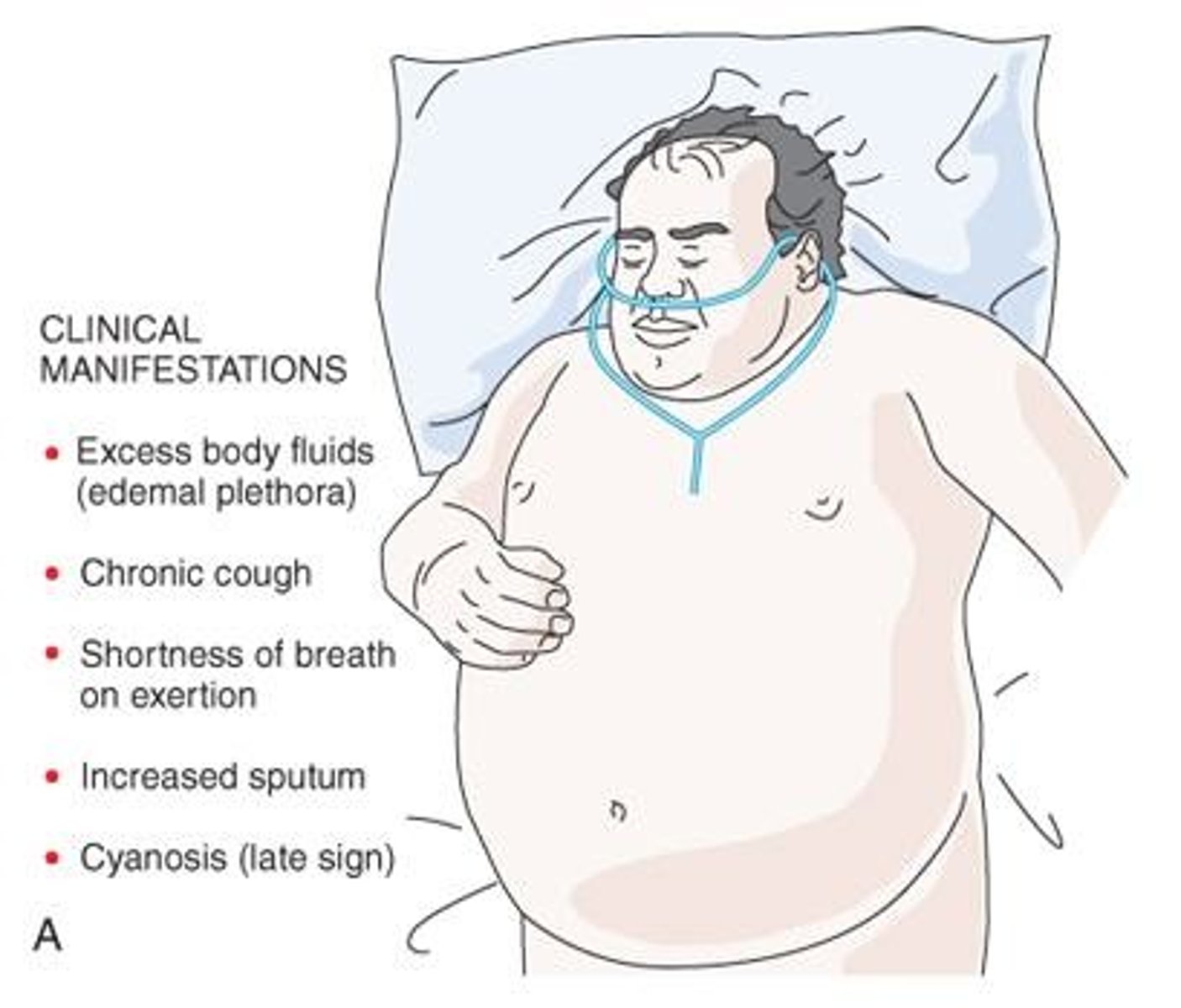
What are typical clinical manifestations of chronic bronchitis?
Overweight patients, shortness of breath on expiration, excessive sputum, chronic cough (more severe in mornings), evidence of excess body fluids (edema, hypervolemia), and cyanosis (late sign).
What is chronic bronchitis also known as?
Type B COPD, or 'blue bloater'.
What diagnostic tests are used for chronic bronchitis?
Chest x-ray showing signs of pulmonary hypertension, pulmonary function tests, arterial blood gas analysis, and ECG.
What findings on a chest x-ray indicate chronic bronchitis?
Increased bronchial vascular markings, congested lung fields, enlarged horizontal cardiac silhouette, and evidence of previous pulmonary infection.
What are the results of pulmonary function tests in chronic bronchitis?
Normal total lung capacity, increased residual volume, decreased FEV1, and decreased FEV1/FVC.
What arterial blood gas results are typical in chronic bronchitis?
Elevated PaCO2 and decreased PO2.
What ECG changes may be seen in chronic bronchitis?
Atrial arrhythmias and evidence of right ventricular hypertrophy.
What is secondary polycythemia in the context of chronic bronchitis?
It is related to continuous or nocturnal hypoxemia.
What are the main management strategies for chronic bronchitis?
Smoking cessation, bronchodilator therapy, reduction of exposure to irritants, and low-dose O2 therapy.
What medications are commonly used in the treatment of chronic bronchitis?
Inhaled short-acting B2 agonists, inhaled anticholinergic bronchodilators, cough suppressants, antimicrobial agents, inhaled/oral corticosteroids, and theophylline products.
What lifestyle changes are recommended for patients with chronic bronchitis?
Adequate rest, proper hydration, physical reconditioning, and vaccination against influenza and pneumococcus.
What is emphysema also known as?
Type A COPD, or 'pink puffer'.
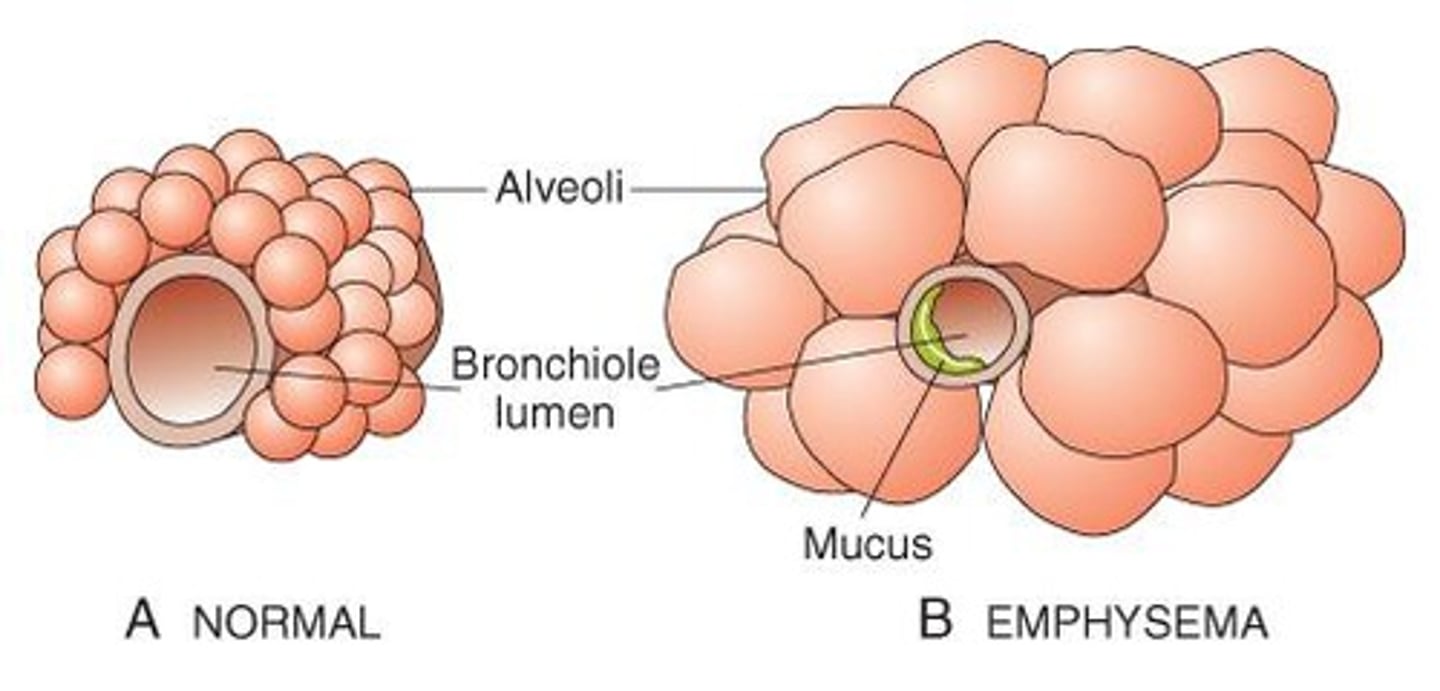
What are the key characteristics of emphysema?
Destructive changes of the alveolar walls without fibrosis and abnormal enlargement of the distal air sacs.
What are the causes of emphysema?
Smoking (more than 70 packs/year), air pollution, certain occupations (mining, welding, asbestos exposure), and α1-antitrypsin deficiency.
How does smoking contribute to the pathogenesis of emphysema?
It causes alveolar damage through inflammation and the release of proteolytic enzymes, while also inactivating α1-antitrypsin.
What role do proteolytic enzymes play in emphysema?
They are released from neutrophils and macrophages, leading to alveolar damage.
What is the impact of α1-antitrypsin on lung health?
It normally protects surfactant, which in turn protects lung parenchyma.
What is a common symptom of emphysema?
Shortness of breath, particularly during exertion.
What is the prognosis for patients with emphysema?
The damage is irreversible, and management focuses on symptom relief and quality of life.
What is the pathogenesis of emphysema?
Loss of elastic tissue in the lung leads to loss of radial traction, trapping air in distal alveoli, causing loss of alveolar wall and formation of bullae, and reduction in pulmonary capillary bed.
What are the clinical manifestations of emphysema?
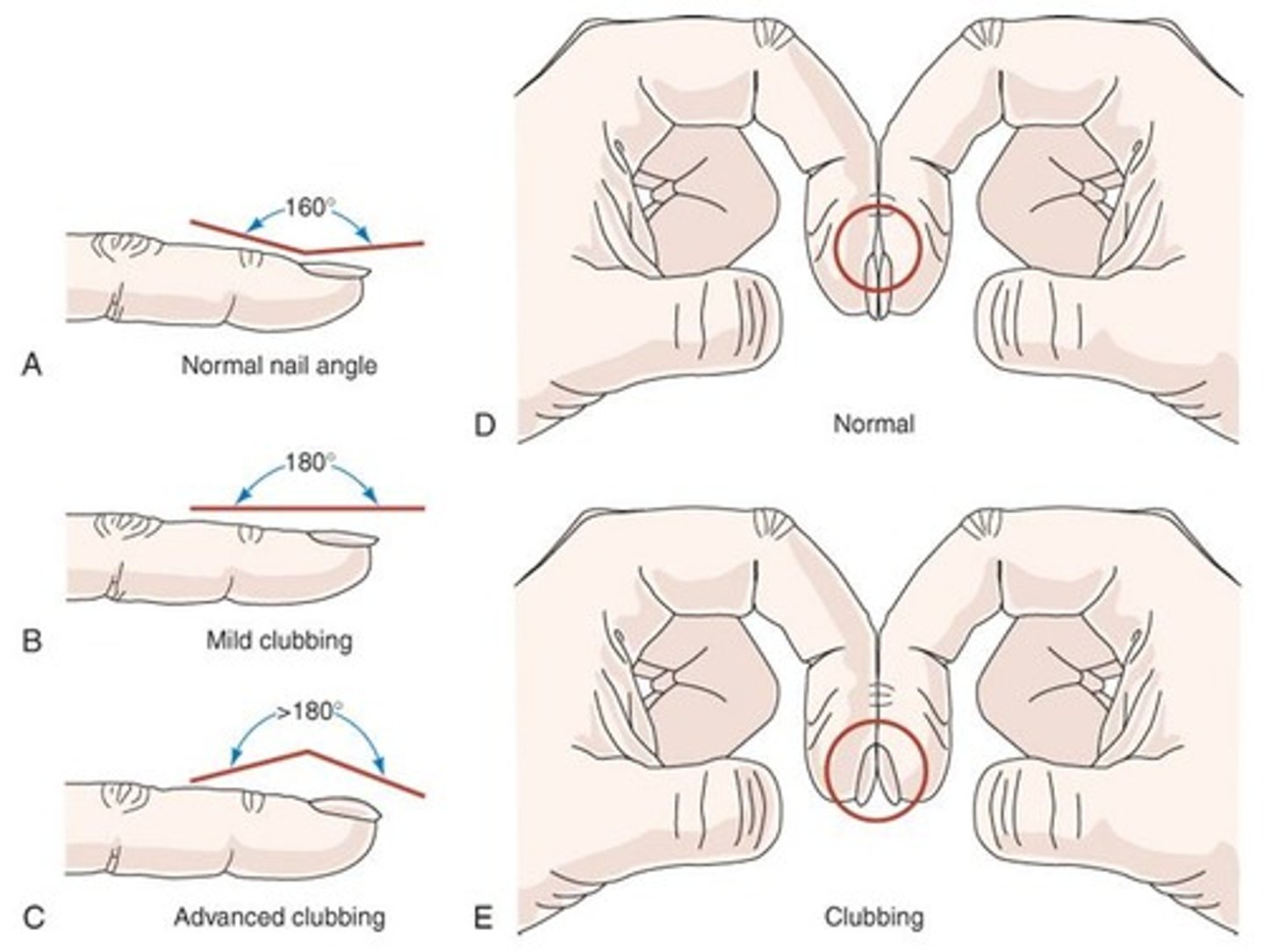
Progressive exertional dyspnea, thin appearance, use of accessory muscles, pursed-lip breathing, minimal or absent cough, digital clubbing, and barrel chest.
What is the alternative name for emphysema in the context of COPD?
Type A COPD, also known as 'Pink puffer'.
What pulmonary function test results are indicative of emphysema?
Increased functional residual capacity, increased RV and TLC, and decreased FEV1 and FVC.
What are the chest x-ray findings in a patient with emphysema?
Hyperventilation, low flat diaphragm, presence of blebs or bullae, narrow mediastinum, and normal or small 'vertical' heart.
What ECG changes may be observed in emphysema patients?
Normal ECG may show tall P waves, sinus tachycardia, supraventricular arrhythmias, and ventricular irregularities.
What are the arterial blood gas (ABG) findings in emphysema?
Mild decrease in PaO2 and normal PaCO2, which may be elevated in late stages.
What common physical findings are associated with emphysema?
Thin, wasted individual hunched forward, using accessory muscles, decreased breath sounds, prolonged expiration, decreased heart sounds, hyperresonance, and decreased diaphragmatic excursion.
What are the treatment options for emphysema?
Oxygen therapy, smoking cessation, inhaled short-acting B2 agonists, inhaled anticholinergic bronchodilators, cough suppressants, antimicrobial agents, inhaled/oral corticosteroids, and theophylline products.
What characterizes restrictive pulmonary disorders?
Decreased lung expansion due to alterations in lung parenchyma, pleura, chest wall, or neuromuscular function.
What are the classifications of restrictive pulmonary disorders?
Pulmonary and extrapulmonary.
What changes in lung volumes are characteristic of restrictive pulmonary disorders?
Decrease in vital capacity (VC), total lung capacity (TLC), functional residual capacity (FRC), and residual volume (RV).
How does the severity of restrictive pulmonary disorders correlate with lung volume decrease?
The greater the decrease in lung volume, the greater the severity of the disease.
What are the ABG findings in restrictive pulmonary disorders?
Decreased PaO2, normal or decreased PaCO2, and increased pH (alkalosis).
What is the etiology of Acute Respiratory Distress Syndrome (ARDS)?
Damage to the alveolar-capillary membrane causes widespread protein-rich alveolar infiltrates, leading to severe dyspnea.
How many cases of ARDS occur annually in the United States?
More than 150,000 cases per year.
What is the mortality rate associated with ARDS?
The mortality rate ranges from 30% to 63%.
What characterizes the decline in PaO2 in ARDS?
The decline in PaO2 is refractory to supplemental oxygen therapy.
What are some common causes of ARDS?
Common causes include severe trauma, sepsis (over 40%), aspiration of gastric acid (over 30%), fat emboli syndrome, and shock.
What are the pathophysiological changes in ARDS?
Increased permeability of the pulmonary vasculature leads to flooding of the alveoli with proteinaceous fluid, resulting in diffuse, fluffy alveolar infiltrates and severe hypoxemia.
What triggers the immune response in ARDS?
The flooding of alveoli triggers the immune system to activate the complement system and initiate neutrophil sequestration in the lung.
What is the impact of ARDS on lung compliance?
ARDS causes atelectasis and a decrease in lung compliance due to a lack of surfactant.
What are the early clinical manifestations of ARDS?
Early symptoms include sudden marked respiratory distress, slight increase in pulse rate, dyspnea, low PaO2, and shallow, rapid breathing.
What are the late clinical manifestations of ARDS?
Late symptoms include tachycardia, tachypnea, hypotension, marked restlessness, frothy secretions, crackles/rhonchi on auscultation, use of accessory muscles, and cyanosis.
What is the hallmark diagnosis of ARDS?
The hallmark is hypoxemia that is refractory to increased levels of supplemental oxygen.
What abnormalities are typically found in arterial blood gas (ABG) results for ARDS?
ABG results typically show hypoxia, acidosis, and hypercapnia.
What does a chest x-ray reveal in ARDS?
A chest x-ray may appear normal initially but can progress to diffuse 'whiteout'.
What pulmonary function test (PFT) results are indicative of ARDS?
PFTs show a decrease in FVC, decreased lung volumes, decreased lung compliance, and VA/Q mismatch with a large right-to-left shunt.
What findings can be observed in an open-lung biopsy for ARDS?
An open-lung biopsy may show atelectasis, hyaline membranes, cellular debris, and interstitial and alveolar edema.
What is the primary approach to treating ARDS?
The treatment is mostly supportive, focusing on maintaining oxygenation and addressing the underlying cause.
What role does inhaled nitric oxide play in ARDS treatment?
Inhaled nitric oxide reduces oxygen usage in the heart and promotes vasodilation to increase tissue oxygenation until inflammation resolves.
What mechanical ventilation strategies are used in ARDS?
Strategies include high-frequency jet ventilation (HFJV), inverse ratio ventilation (IRV), volume ventilation with pressure support, and mechanical ventilation with positive end-expiratory pressure (PEEP).
How does PEEP benefit patients with ARDS?
PEEP increases functional residual volume (FRV) and prevents alveolar collapse at end-expiration, forcing edema out of the alveoli.
What is important to maintain during ARDS treatment?
Maintaining fluid and electrolyte balance is crucial, as increased fluid administration can worsen pulmonary edema.