Kp- Equilibrium constant
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
What is partial pressure?
The partial pressure of a gas in a mixture is the pressure that the gas would have occupied if it alone occupied the volume occupied by the whole mixture.
If a mixture of gases contain 3 different gases then the total pressure will equal the 3 partial pressures added together.
PP- Mole fraction x Total pressure
p1 = x1 P
Mole fraction
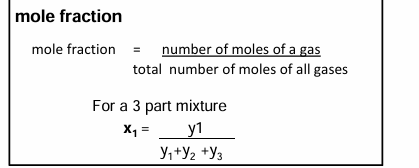
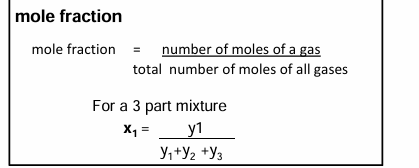
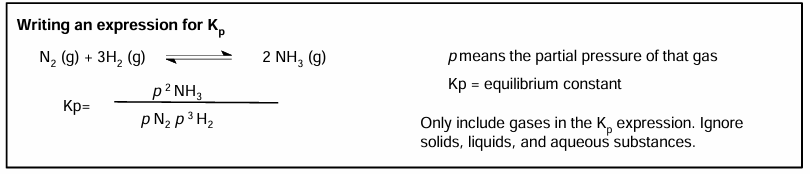
Writing an expression for Kp
Brackets on outside

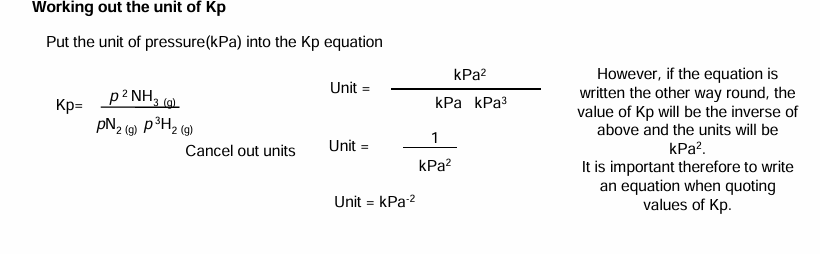
Working out the unit of Kp

What is homogenous equilibria?
When all substances are in the same state.
Kp can determine which side equilibrium favours
Heterogenous equilibria for Kp
Kp expressions only contain gaseous substances. Any substance with another state is left out.
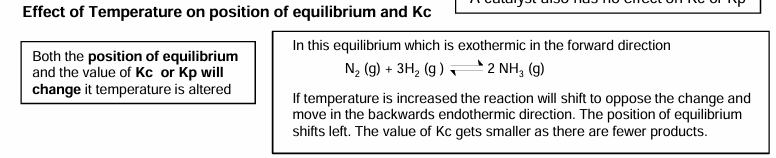
Effect of changing conditions on value of Kc or Kp
The larger the Kc, the greater the amount of products.
If Kc is small we say equilibrium favours the reactants.
They only change with temperature.
Temperature on position on equilibrium

Effect of pressure on position of equilibrium and Kp


Why does pressure not effect Kp?
Increasing pressure does not change Kp. The increased pressure increases the pressure terms on bottom of Kp expression more than the top. The system is now no longer in equilibrium so the equilibrium shifts to the right increasing mole fractions of products and decreases the mole fractions of reactants. The top of Kp expression therefore increases and the bottom decreases until the original value of Kp is restored